The Brain and Behavior: an Introduction to Behavioral
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Creation of Neuroscience
The Creation of Neuroscience The Society for Neuroscience and the Quest for Disciplinary Unity 1969-1995 Introduction rom the molecular biology of a single neuron to the breathtakingly complex circuitry of the entire human nervous system, our understanding of the brain and how it works has undergone radical F changes over the past century. These advances have brought us tantalizingly closer to genu- inely mechanistic and scientifically rigorous explanations of how the brain’s roughly 100 billion neurons, interacting through trillions of synaptic connections, function both as single units and as larger ensem- bles. The professional field of neuroscience, in keeping pace with these important scientific develop- ments, has dramatically reshaped the organization of biological sciences across the globe over the last 50 years. Much like physics during its dominant era in the 1950s and 1960s, neuroscience has become the leading scientific discipline with regard to funding, numbers of scientists, and numbers of trainees. Furthermore, neuroscience as fact, explanation, and myth has just as dramatically redrawn our cultural landscape and redefined how Western popular culture understands who we are as individuals. In the 1950s, especially in the United States, Freud and his successors stood at the center of all cultural expla- nations for psychological suffering. In the new millennium, we perceive such suffering as erupting no longer from a repressed unconscious but, instead, from a pathophysiology rooted in and caused by brain abnormalities and dysfunctions. Indeed, the normal as well as the pathological have become thoroughly neurobiological in the last several decades. In the process, entirely new vistas have opened up in fields ranging from neuroeconomics and neurophilosophy to consumer products, as exemplified by an entire line of soft drinks advertised as offering “neuro” benefits. -

The Baseline Structure of the Enteric Nervous System and Its Role in Parkinson’S Disease
life Review The Baseline Structure of the Enteric Nervous System and Its Role in Parkinson’s Disease Gianfranco Natale 1,2,* , Larisa Ryskalin 1 , Gabriele Morucci 1 , Gloria Lazzeri 1, Alessandro Frati 3,4 and Francesco Fornai 1,4 1 Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, 56126 Pisa, Italy; [email protected] (L.R.); [email protected] (G.M.); [email protected] (G.L.); [email protected] (F.F.) 2 Museum of Human Anatomy “Filippo Civinini”, University of Pisa, 56126 Pisa, Italy 3 Neurosurgery Division, Human Neurosciences Department, Sapienza University of Rome, 00135 Rome, Italy; [email protected] 4 Istituto di Ricovero e Cura a Carattere Scientifico (I.R.C.C.S.) Neuromed, 86077 Pozzilli, Italy * Correspondence: [email protected] Abstract: The gastrointestinal (GI) tract is provided with a peculiar nervous network, known as the enteric nervous system (ENS), which is dedicated to the fine control of digestive functions. This forms a complex network, which includes several types of neurons, as well as glial cells. Despite extensive studies, a comprehensive classification of these neurons is still lacking. The complexity of ENS is magnified by a multiple control of the central nervous system, and bidirectional communication between various central nervous areas and the gut occurs. This lends substance to the complexity of the microbiota–gut–brain axis, which represents the network governing homeostasis through nervous, endocrine, immune, and metabolic pathways. The present manuscript is dedicated to Citation: Natale, G.; Ryskalin, L.; identifying various neuronal cytotypes belonging to ENS in baseline conditions. -

A Revised Computational Neuroanatomy for Motor Control
This is the author’s final version; this article has been accepted for publication in the Journal of Cognitive Neuroscience 1 A Revised Computational Neuroanatomy for Motor Control 2 Shlomi Haar1, Opher Donchin2,3 3 1. Department of BioEngineering, Imperial College London, UK 4 2. Department of Biomedical Engineering, Ben-Gurion University of the Negev, Israel 5 3. Zlotowski Center for Neuroscience, Ben-Gurion University of the Negev, Israel 6 7 Corresponding author: Shlomi Haar ([email protected]) 8 Imperial College London, London, SW7 2AZ, UK 9 10 Acknowledgements: We would like to thank Ilan Dinstein, Liad Mudrik, Daniel Glaser, Alex Gail, and 11 Reza Shadmehr for helpful discussions about the manuscript. Shlomi Haar is supported by the Royal 12 Society – Kohn International Fellowship (NF170650). Work on this review was partially supported by 13 DFG grant TI-239/16-1. 14 15 Abstract 16 We discuss a new framework for understanding the structure of motor control. Our approach 17 integrates existing models of motor control with the reality of hierarchical cortical processing and the 18 parallel segregated loops that characterize cortical-subcortical connections. We also incorporate the recent 19 claim that cortex functions via predictive representation and optimal information utilization. Our 20 framework assumes each cortical area engaged in motor control generates a predictive model of a different 21 aspect of motor behavior. In maintaining these predictive models, each area interacts with a different part 22 of the cerebellum and basal ganglia. These subcortical areas are thus engaged in domain appropriate 23 system identification and optimization. This refocuses the question of division of function among different 24 cortical areas. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

A Cyclops and a Synotus by K
J Neurol Psychopathol: first published as 10.1136/jnnp.s1-17.65.48 on 1 July 1936. Downloaded from 48 ORIGINAL PAPERS A CYCLOPS AND A SYNOTUS BY K. H. BOUMAN, AMSTERDAM, AND V. W. D. SCHENK, TiH HAGUE INTRODUCTION ONLY a small number of cases of cyclopia in human beings and mammals have been minutely examined. The number becomes still smaller if a more or less complete microscopic investigation of the central nervous system is stipulated. It is really only the cases of Davidson Black and Winkler and perhaps that of Naegli which answer this requirement. In contrast therewith there is an abundance of experimental studies in this field in urodela and other lower classes of animals. For all that, unanimity does not by any means prevail here, although the Protected by copyright. views of Stockard and his followers-who held that the first determination of the eye lay unpaired in the median line-and those of Spemann-who pointed to a paired rudiment from the outset, which views were originally diametrically opposed-appear to have drawn somewhat nearer to each other in recent years. Woerdeman, for instance, found that the paired rudiment of the eye shifts its position laterally downwards very early (when the folds of the medullary plate become visible) and he rightly says that this is not the same as Stockard's lateral growth of an unpaired eye rudiment. Yet, by saying this, he admits certain changes and growth conditions to which Fischel, for instance, did not do full justice. E. Manchot, on the other hand, who defends Stockard's views, admits that between the two regions of the eye http://jnnp.bmj.com/ rudiment there must be a tract of brain tissue (lamina terminalis and regio chiasmatica). -
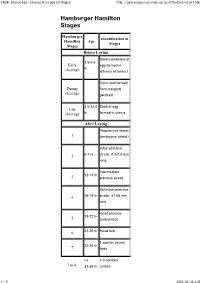
Hamburger Hamilton Stages
UNSW Embryology- Chicken Development Stages http://embryology.med.unsw.edu.au/OtherEmb/chick1.htm Hamburger Hamilton Stages Hamburger Identification of Hamilton Age Stages Stages Before Laying Shell membrane of 3.5-4.5 Early egg formed in hr cleavage isthmus of oviduct Germ wall formed During from marginal cleavage periblast 4.5-24.0 Shell of egg Late cleavage hr formed in uterus After Laying Preprimitive streak 1 (embryonic shield) Initial primitive 2 6-7 hr streak, 0.3-0.5 mm long Intermediate 12-13 hr 3 primitive streak Definitive primitive 4 18-19 hr streak, ±1.88 mm long Head process 19-22 hr 5 (notochord) 6 23-25 hr Head fold 1 somite; neural 23-26 hr 7 folds ca. 1-3 somites; 7 to 8- 23-26 hr coelom 1 / 5 2007/03/20 9:05 UNSW Embryology- Chicken Development Stages http://embryology.med.unsw.edu.au/OtherEmb/chick1.htm 4 somites; blood 26-29 hr 8 islands 7 somites; primary 29-33 hr 9 optic vesicles 8-9 somites; 9+ to 10- ca. 33 hr anterior amniotic fold 10 somites; 3 10 33-38 hr primary brain vesicles 13 somites; 5 11 40-45 hr neuromeres of hindbrain 16 somites; 45-49 hr 12 telencephalon 19 somites; 13 48-52 hr atrioventricular canal ca. 20-21 somites; tail 13+ to 14- 50-52 hr bud 22 somites; trunk flexure; visceral 50-53 hr 14 arches I and II, clefts 1 and 2 23 somites; ca. premandibular 14+ to 15- 50-54 hr head cavities 24-27 somites; 15 50-55 hr visceral arch III, cleft 3 26-28 somites; 16 51-56 hr wing bud; posterior 2 / 5 2007/03/20 9:05 UNSW Embryology- Chicken Development Stages http://embryology.med.unsw.edu.au/OtherEmb/chick1.htm -

Quantity Determination and the Distance Effect with Letters, Numbers, and Shapes: a Functional MR Imaging Study of Number Processing
AJNR Am J Neuroradiol 23:193–200, February 2003 Quantity Determination and the Distance Effect with Letters, Numbers, and Shapes: A Functional MR Imaging Study of Number Processing Robert K. Fulbright, Stephanie C. Manson, Pawel Skudlarski, Cheryl M. Lacadie, and John C. Gore BACKGROUND AND PURPOSE: The ability to quantify, or to determine magnitude, is an important part of number processing, and the extent to which language and other cognitive abilities are involved with number processing is an area of interest. We compared activation patterns, reaction times, and accuracy as subjects determined stimulus magnitude by ordering letters, numbers, and shapes. A second goal was to define the brain regions involved in the distance effect (the farther apart numbers are, the faster subjects are at judging which number is larger) and whether this effect depended on stimulus type. METHODS: Functional MR images were acquired in 19 healthy subjects. The order task required the subjects to judge whether three stimuli were in order according to their position in the alphabet (letters), position in the number line (numbers), or size (shapes). In the control (identify task), subjects judged whether one of the three stimuli was a particular letter, number, or shape. Each stimulus type was divided into near trials (quantity difference of three or less) and far trials (quantity difference of at least five) to assess the distance effect. RESULTS: Subjects were less accurate and slower with letters than with numbers and shapes. A distance effect was present with shapes and numbers, as subjects ordered the near trials slower than far trials. No distance effect was detected with letters. -

Basic Brain Anatomy
Chapter 2 Basic Brain Anatomy Where this icon appears, visit The Brain http://go.jblearning.com/ManascoCWS to view the corresponding video. The average weight of an adult human brain is about 3 pounds. That is about the weight of a single small To understand how a part of the brain is disordered by cantaloupe or six grapefruits. If a human brain was damage or disease, speech-language pathologists must placed on a tray, it would look like a pretty unim- first know a few facts about the anatomy of the brain pressive mass of gray lumpy tissue (Luria, 1973). In in general and how a normal and healthy brain func- fact, for most of history the brain was thought to be tions. Readers can use the anatomy presented here as an utterly useless piece of flesh housed in the skull. a reference, review, and jumping off point to under- The Egyptians believed that the heart was the seat standing the consequences of damage to the structures of human intelligence, and as such, the brain was discussed. This chapter begins with the big picture promptly removed during mummification. In his and works down into the specifics of brain anatomy. essay On Sleep and Sleeplessness, Aristotle argued that the brain is a complex cooling mechanism for our bodies that works primarily to help cool and The Central Nervous condense water vapors rising in our bodies (Aristo- tle, republished 2011). He also established a strong System argument in this same essay for why infants should not drink wine. The basis for this argument was that The nervous system is divided into two major sec- infants already have Central nervous tions: the central nervous system and the peripheral too much moisture system The brain and nervous system. -

Nervous System Cns
THE NERVOUS SYSTEM CNS • Function The Spinal Cord • General Structure • Enclosed In: • Neural Foramen – length • Connects With: • Foramen magnum – need for protection Coverings • Coverings: • meninges (3) • Subarachnoid space • location • composition • diagnostic use Spinal Nerves • Caudal equina Finer Structures of Spinal Cord • Gray Matter • composition • function • horns (2) • horns form roots (2) • gray commisure • central canal Finer Structures of Spinal Cord • White matter • arrangement • columns contain tracts • description • names The Brain •General Structure •Protection •Skeletal •Membranous The Brain • Development • Neural Plate • Neural Tube and 3 swellings The Brain • Other Structures • Ventricles • Foramen of Monroe • Cerebral Aqueduct The Brain - finer structures • Brain Stem • Medulla • location • connection • gray matter vs. white matter • function • kinds of reflexes The Brain • Brain stem • Pons • Structure • Composition • Nerves The Brain • Brain stem • Midbrain • Location • Composition: • Cerebral peduncles • Substantia nigra • Tegmentum • Corpora quadrigemina • Cerebral aqueduct The Brain • Cerebellum • Location • Structure • Cortex The Brain • Cerebellum • White Matter • Cerebellar Nuclei • Dentate Nuclei • Furrows • Divisions • Functions The Brain • Interbrain • Contains structures (2) • Location • How functions were determined Interbrain • The Thalamus • Function • Result of Injury Interbrain • The Hypothalmus • Function • Reason for these functions • Result of Injury The Brain • The Cerebrum • Size • Complexity • -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

Metabolic Responses in Discrete, Mice Brain Regions Like CC, CG, H and CQ During PTZ Induced Epileptic Seizures
Current Neurobiology 2012; 3 (1): 31-38 ISSN 0975-9042 Scientific Publishers of India Metabolic responses in discrete, Mice brain regions like CC, CG, H and CQ during PTZ induced epileptic seizures. K. K. Therisa and P.V. Desai Department of Zoology, Goa University, Taleigao Plateau, Goa 403206, India. Abstract Epilepsy, a neurological disorder with recurrent seizures, involves disruption of different metabolic enzymes and its related metabolites altering the normal processes of metabolism, either during the onset or post epilepsy in the brain. In the present work, it is convincingly, observed that the Mice brain regions such as Corpus callosum (CC), Cingulate gyrus (CG), Hippocampus (H) and Corpora quadrigemina (CQ) shows significant changes in the activi- ties of metabolic enzymes such as AST, ALT, LDH; ATPases like Na +, K +-ATPase, Mg 2+ - ATPase, Ca 2+ -ATPase along with their metabolites such as Glucose, Pyruvate, Lactate and Glutamate, altering the metabolic integrity during Pentylenetetrazole (PTZ) induced epilep- tic seizure. We report from the present work that, H and CG are affected completely during epileptic seizure as compared to its control but CC and CQ shows partly altered as far as metabolism in brain is concerned. Keywords: Corpus callosum, Hippocampus, Cingulate gyrus, Corpora Quadrigemina, Metabolic enzymes and metabo- lites. Accepted April 07 2012 Introduction may overlap with other frontal lobe epilepsy syndromes. But, an aberrant behaviors observed in epileptic patients Epilepsy, involves a disruption of brain energy homeosta- completely resolved after lesionectomy of Cingulate sis and is potentially manageable through principles of gyrus [6]. Hippocampus and Cingulate gyrus forms the metabolic control theory. -

Common and Distinct Functional Brain Networks for Intuitive and Deliberate Decision Making
brain sciences Article Common and Distinct Functional Brain Networks for Intuitive and Deliberate Decision Making Burak Erdeniz 1,* and John Done 2 1 Department of Psychology, Izmir˙ University of Economics, 35330 Izmir, Turkey 2 Department of Psychology and Sports Sciences, School of Life and Medical Sciences, University of Hertfordshire, Hatfield AL 10 9AB, UK * Correspondence: [email protected]; Tel.: +902-324-888-379 Received: 5 June 2019; Accepted: 19 July 2019; Published: 20 July 2019 Abstract: Reinforcement learning studies in rodents and primates demonstrate that goal-directed and habitual choice behaviors are mediated through different fronto-striatal systems, but the evidence is less clear in humans. In this study, functional magnetic resonance imaging (fMRI) data were collected whilst participants (n = 20) performed a conditional associative learning task in which blocks of novel conditional stimuli (CS) required a deliberate choice, and blocks of familiar CS required an intuitive choice. Using standard subtraction analysis for fMRI event-related designs, activation shifted from the dorso-fronto-parietal network, which involves dorsolateral prefrontal cortex (DLPFC) for deliberate choice of novel CS, to ventro-medial frontal (VMPFC) and anterior cingulate cortex for intuitive choice of familiar CS. Supporting this finding, psycho-physiological interaction (PPI) analysis, using the peak active areas within the PFC for novel and familiar CS as seed regions, showed functional coupling between caudate and DLPFC when processing novel CS and VMPFC when processing familiar CS. These findings demonstrate separable systems for deliberate and intuitive processing, which is in keeping with rodent and primate reinforcement learning studies, although in humans they operate in a dynamic, possibly synergistic, manner particularly at the level of the striatum.