Macro-Ecological Patterns in Seed Removal by Animals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Synopsis of Phaseoleae (Leguminosae, Papilionoideae) James Andrew Lackey Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1977 A synopsis of Phaseoleae (Leguminosae, Papilionoideae) James Andrew Lackey Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Botany Commons Recommended Citation Lackey, James Andrew, "A synopsis of Phaseoleae (Leguminosae, Papilionoideae) " (1977). Retrospective Theses and Dissertations. 5832. https://lib.dr.iastate.edu/rtd/5832 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -

Flora.Sa.Gov.Au/Jabg
JOURNAL of the ADELAIDE BOTANIC GARDENS AN OPEN ACCESS JOURNAL FOR AUSTRALIAN SYSTEMATIC BOTANY flora.sa.gov.au/jabg Published by the STATE HERBARIUM OF SOUTH AUSTRALIA on behalf of the BOARD OF THE BOTANIC GARDENS AND STATE HERBARIUM © Board of the Botanic Gardens and State Herbarium, Adelaide, South Australia © Department of Environment, Water and Natural Resources, Government of South Australia All rights reserved State Herbarium of South Australia PO Box 2732 Kent Town SA 5071 Australia © 2012 Board of the Botanic Gardens & State Herbarium, Government of South Australia J. Adelaide Bot. Gard. 25 (2012) 71–96 © 2012 Department of Environment, Water and Natural Resources, Govt of South Australia Notes on Hibbertia (Dilleniaceae) 8. Seven new species, a new combination and four new subspecies from subgen. Hemistemma, mainly from the central coast of New South Wales H.R. Toelkena & R.T. Millerb a State Herbarium of South Australia, DENR Science Resource Centre, P.O. Box 2732, Kent Town, South Australia 5071 E-mail: [email protected] b 13 Park Road, Bulli, New South Wales 2516 E-mail: [email protected] Abstract Increased collections from the Hibbertia-rich vicinity of Sydney, New South Wales, prompted a survey of rarer species to publicise the need for more information ahead of the rapid urban spread. Many of these species were previously misunderstood or are listed as rare and endangered. Thirteen new taxa (in bold) are described and discussed in context with the following seventeen taxa within seven different species groups: 1. H. acicularis group: H. woronorana Toelken; 2. H. humifusa group: H. -
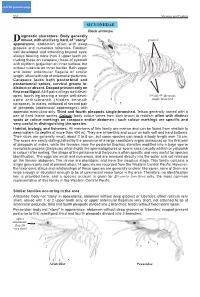
W7192e19.Pdf
click for previous page 952 Shrimps and Prawns Sicyoniidae SICYONIIDAE Rock shrimps iagnostic characters: Body generally Drobust, with shell very hard, of “stony” grooves appearance; abdomen often with deep grooves and numerous tubercles. Rostrum well developed and extending beyond eyes, always bearing more than 3 upper teeth (in- cluding those on carapace); base of eyestalk with styliform projection on inner surface, but without tubercle on inner border. Both upper and lower antennular flagella of similar length, attached to tip of antennular peduncle. 1 Carapace lacks both postorbital and postantennal spines, cervical groove in- distinct or absent. Exopod present only on first maxilliped. All 5 pairs of legs well devel- 2 oped, fourth leg bearing a single well-devel- 3rd and 4th pleopods 4 single-branched oped arthrobranch (hidden beneath 3 carapace). In males, endopod of second pair 5 of pleopods (abdominal appendages) with appendix masculina only. Third and fourth pleopods single-branched. Telson generally armed with a pair of fixed lateral spines. Colour: body colour varies from dark brown to reddish; often with distinct spots or colour markings on carapace and/or abdomen - such colour markings are specific and very useful in distinguishing the species. Habitat, biology, and fisheries: All members of this family are marine and can be found from shallow to deep waters (to depths of more than 400 m). They are all benthic and occur on both soft and hard bottoms. Their sizes are generally small, about 2 to 8 cm, but some species can reach a body length over 15 cm. The sexes are easily distinguished by the presence of a large copulatory organ (petasma) on the first pair of pleopods of males, while the females have the posterior thoracic sternites modified into a large sperm receptacle process (thelycum) which holds the spermatophores or sperm sacs (usually whitish or yellowish in colour) after mating. -

Cassytha Pubescens
Cassytha pubescens: Germination biology and interactions with native and introduced hosts Hong Tai (Steven), Tsang B.Sc. Hons (University of Adelaide) Thesis submitted for the degree of Master of Science School of Earth & Environmental Science University of Adelaide, Australia 03/05/2010 i Table of Contents Table of Contents ........................................................................................................... ii Abstract .......................................................................................................................... v Declaration ................................................................................................................... vii Acknowledgements .................................................................................................... viii Chapter. 1 Introduction .................................................................................................. 1 1.1 General Introduction ............................................................................................ 1 1.2 Literature Review ................................................................................................. 3 1.2.1 Characteristics of parasitic control agents .................................................... 3 1.2.2. Direct impacts on hosts ................................................................................ 7 1.2.3. Indirect impacts on hosts ............................................................................. 8 1.2.4. Summary ................................................................................................... -

A Reappraisal of Phylogenetic Relationships Among Auchenipterid Catfishes of the Subfamily Centromochlinae and Diagnosis of Its Genera (Teleostei: Siluriformes)
ISSN 0097-3157 PROCEEDINGS OF THE ACADEMY OF NATURAL SCIENCES OF PHILADELPHIA 167: 85-146 2020 A reappraisal of phylogenetic relationships among auchenipterid catfishes of the subfamily Centromochlinae and diagnosis of its genera (Teleostei: Siluriformes) LUISA MARIA SARMENTO-SOARES Programa de Pós-Graduação em Biologia Animal, Universidade Federal do Espírito Santo. Prédio Bárbara Weinberg, Campus de Goiabeiras, 29043-900, Vitória, ES, Brasil. http://orcid.org/0000-0002-8621-1794 Laboratório de Ictiologia, Universidade Estadual de Feira de Santana. Av. Transnordestina s/no., Novo Horizonte, 44036-900, Feira de Santana, BA, Brasil Instituto Nossos Riachos, INR, Estrada de Itacoatiara, 356 c4, 24348-095, Niterói, RJ. www.nossosriachos.net E-mail: [email protected] RONALDO FERNANDO MARTINS-PINHEIRO Instituto Nossos Riachos, INR, Estrada de Itacoatiara, 356 c4, 24348-095, Niterói, RJ. www.nossosriachos.net E-mail: [email protected] ABSTRACT.—A hypothesis of phylogenetic relationships is presented for species of the South American catfish subfamily Centromochlinae (Auchenipteridae) based on parsimony analysis of 133 morphological characters in 47 potential ingroup taxa and one outgroup taxon. Of the 48 species previously considered valid in the subfamily, only one, Centromochlus steindachneri, was not evaluated in the present study. The phylogenetic analysis generated two most parsimonious trees, each with 202 steps, that support the monophyly of Centromochlinae composed of five valid genera: Glanidium, Gephyromochlus, Gelanoglanis, Centromochlus and Tatia. Although those five genera form a clade sister to the monotypic Pseudotatia, we exclude Pseudotatia from Centromochlinae. The parsimony analysis placed Glanidium (six species) as the sister group to all other species of Centromochlinae. Gephyromochlus contained a single species, Gephyromochlus leopardus, that is sister to the clade Gelanoglanis (five species) + Centromochlus (eight species). -

Contents About This Booklet 2 1
Contents About this booklet 2 1. Why indigenous gardening? 3 Top ten reasons to use indigenous plants 3 Indigenous plants of Whitehorse 4 Where can I buy indigenous plants of Whitehorse? 4 2. Sustainable Gardening Principles 5 Make your garden a wildlife garden 6 3. Tips for Successful Planting 8 1. Plant selection 8 2. Pre-planting preparation 10 3. Planting technique 12 4. Early maintenance 14 4. Designing your Garden 16 Climbers 16 Hedges and borders 17 Groundcovers and fillers 17 Lawn alternatives 18 Feature trees 18 Screen plants 19 Damp & shady spots 19 Edible plants 20 Colourful flowers 21 5. 94 Species Indigenous to Whitehorse 23 6. Weeds of Whitehorse 72 7. Further Resources 81 8. Index of Plants 83 Alphabetically by Botanical Name 83 Alphabetically by Common Name 85 9. Glossary 87 1 In the spirit of About this booklet reconciliation, Whitehorse City Council This booklet has been written by Whitehorse acknowledges the City Council to help gardeners and landscapers Wurundjeri people as adopt sustainable gardening principles by using the traditional owners indigenous plants commonly found in Whitehorse. of the land now known The collective effort of residents gardening with as Whitehorse and pays indigenous species can make a big difference to respects to its elders preserving and enhancing our biodiversity. past and present. We would like to acknowledge the volunteers of the Blackburn & District Tree Preservation Society, Whitehorse Community Indigenous Plant Project Inc. (Bungalook Nursery) and Greenlink Box Hill Nursery for their efforts to protect and enhance the indigenous flora of Whitehorse. Information provided by these groups is included in this guide. -
Abelmoschus Moschatus Subsp
Cooktown Botanic Gardens Index Plantarum 2011 Family Published Taxon Name Plate No Acanthaceae Eranthemum pulchellum Andrews 720 Acanthaceae Graptophyllum excelsum (F.Meull.) Druce 515 Acanthaceae Graptophyllum spinigerum (F.Meull.) 437 Acanthaceae Megaskepasma erythrochlamys Lindau 107 Acanthaceae Pseuderanthemum variabile (R.Br.) Radlk. 357 Adiantaceae Adiantum formosum R.Br. 761 Adiantaceae Adiantum hispidulum Sw. 762 Adiantaceae Adiantum philippense L. 765 Adiantaceae Adiantum silvaticum Tindale 763 Adiantaceae Adiantum Walsh River 764 Agavaceae Beaucarnea recurvata Lem. 399 Agavaceae Furcraea foetida (L.) Haw. 637 Agavaceae Furcraea gigantea (L.) Haw. 049 Agavaceae Yucca elephantipes Hort.ex Regel 388 Agavaceae Agave sisalana Perrine. 159 Amarylidaceae Scadoxus Raf. sp 663 Amaryllidacea, Crinum angustifolium R.Br. 536 Liliaceae Amaryllidacea, Crinum asiaticum var. procerum (Herb. et Carey) Baker 417 Liliaceae Amaryllidacea, Crinum pedunculatum R.Br. 265 Liliaceae Amaryllidacea, Crinum uniflorum F.Muell. 161 Liliaceae Amaryllidaceae Hymenocallis Salisb. americanus 046 Amaryllidaceae Hymenocallis Salisb. peruvianna 045 Amaryllidaceae Proiphys amboinensis (L.) Herb. 041 Anacardiaceae Anacardium occidentale L. 051 Anacardiaceae Buchanania arborescens (Blume) Blume. 022 Anacardiaceae Euroschinus falcatus Hook.f. var. falcatus 429 Anacardiaceae Mangifera indica L. 009 Anacardiaceae Pleiogynium timorense (DC.) Leenh. 029 Anacardiaceae Semecarpus australiensis Engl. 368 Annonaceae Annona muricata L. 054 Annonaceae Annona reticulata L. 053 Annonaceae Annona squamosa 602 Annonaceae Cananga odorata (Lam.) Hook.f.&Thomson 406 Annonaceae Melodorum leichhardtii (F.Muell.) Diels. 360 Annonaceae Rollinia deliciosa Saff. 098 Apiaceae Centella asiatica (L.) Urb. 570 Apocynaceae Adenium obesum (Forssk.) Roem. & Schult. 489 Apocynaceae Allamanda cathartica L. 047 Apocynaceae Allamanda violacea Gardn. & Field. 048 Apocynaceae Alstonia actinophylla (A.Cunn.) K.Schum. 026 Apocynaceae Alstonia scholaris (L.) R.Br. 012 Apocynaceae Alyxia ruscifolia R.Br. -

Australian Native Plants Society Australia Hakea
AUSTRALIAN NATIVE PLANTS SOCIETY AUSTRALIA HAKEA STUDY GROUP NEWSLETTER No. 65 OCTOBER 2017 ISSN0727- 7008 Leader: Paul Kennedy 210 Aireys Street Elliminyt Vic. 3250 E mail [email protected] Tel. 03-52315569 Dear members. We have had a very cold winter and now as spring emerges the cold remains with very wet conditions. Oh how I long for some warm sunshine to brighten our day. However the Hakeas have stood up to the cold weather very well and many have now flowered. Rainfall in August was 30mm but in the first 6 days of September another 56mm was recorded making the soil very moist indeed. The rain kept falling in September with 150mm recorded. Fortunately my drainage work of spoon drains and deeper drains with slotted pipe with blue metal cover on top shed a lot of water straight into the Council drains. Most of the Hakeas like well drained conditions, so building up beds and getting rid of excess water will help in making them survive. The collection here now stands at 162 species out of a possible 169. Seed of some of the remaining species hopefully will arrive here before Christmas so that I can propagate them over summer. Wanderings. Barbara and I spent most of June and July in northern NSW and Queensland to escape the cold conditions down here. I did look around for Hakeas and visited some members’ gardens. Just to the east of Cann River I found Hakea decurrens ssp. physocarpa, Hakea ulicina and Hakea teretifolia ssp. hirsuta all growing on the edge of a swamp. -

Review of Palaemoninae (Crustacea: Decapoda: Caridea) from Vietnam, Macrobrachium Excepted
^CRUSTACEA LIBRARY SMITHSONIAN INST* ftSXURN ZO W-119 Review of Palaemoninae (Crustacea: Decapoda: Caridea) from Vietnam, Macrobrachium excepted Nguyen Van Xuan Nguyen Van Xuan. Review of Palaemoninae (Crustacea: Decapoda: Caridea) from Vietnam, Macrobrachium excepted. Zool. Med. Leiden 66 (2), 31.vii.1992:19-47, figs. 1-12. — ISSN 0024-0672. Key words: Palaemoninae; Leandrites; Leptocarpus; Exopalaemon; Palaemon; new species; Vietnam. An account is presented of the species of Palaemoninae known from Vietnam, the genus Macro- brachium excluded. Of each species a description or descriptive notes are provided, and the habitat and economic importance are discussed. Illustrations of each species, two of which are new to science, are given. Nguyen Van Xuan, Department of Fishery, University of Agriculture and Forestry, Thu Due, Ho Chi Minh Ville, Vietnam. Introduction Very little has been published so far on the Palaemoninae of Vietnam, and most of the species from the area mentioned in the literature belong to the genus Macro- brachium Bate, 1868. Still Palaemoninae are not rare there, and some are even of eco- nomic interest. The present paper is the result of about 20 years of collecting and studying the Palaemonid prawns of Vietnam by the author, and deals with the Palaemoninae, with the exception of the genus Macrobrachium, to which a special study will be devoted. It intends to give an annotated catalogue of all the species known from the area with a description and illustration of their characters, and the variability of these. Apart from an enumeration of the material examined, a discussion is provided of occurrence, ecology, biology and economic importance of the species. -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Phylogenetic Relationships of the South American Doradoidea (Ostariophysi: Siluriformes)
Neotropical Ichthyology, 12(3): 451-564, 2014 Copyright © 2014 Sociedade Brasileira de Ictiologia DOI: 10.1590/1982-0224-20120027 Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes) José L. O. Birindelli A phylogenetic analysis based on 311 morphological characters is presented for most species of the Doradidae, all genera of the Auchenipteridae, and representatives of 16 other catfish families. The hypothesis that was derived from the six most parsimonious trees support the monophyly of the South American Doradoidea (Doradidae plus Auchenipteridae), as well as the monophyly of the clade Doradoidea plus the African Mochokidae. In addition, the clade with Sisoroidea plus Aspredinidae was considered sister to Doradoidea plus Mochokidae. Within the Auchenipteridae, the results support the monophyly of the Centromochlinae and Auchenipterinae. The latter is composed of Tocantinsia, and four monophyletic units, two small with Asterophysus and Liosomadoras, and Pseudotatia and Pseudauchenipterus, respectively, and two large ones with the remaining genera. Within the Doradidae, parsimony analysis recovered Wertheimeria as sister to Kalyptodoras, composing a clade sister to all remaining doradids, which include Franciscodoras and two monophyletic groups: Astrodoradinae (plus Acanthodoras and Agamyxis) and Doradinae (new arrangement). Wertheimerinae, new subfamily, is described for Kalyptodoras and Wertheimeria. Doradinae is corroborated as monophyletic and composed of four groups, one including Centrochir and Platydoras, the other with the large-size species of doradids (except Oxydoras), another with Orinocodoras, Rhinodoras, and Rhynchodoras, and another with Oxydoras plus all the fimbriate-barbel doradids. Based on the results, the species of Opsodoras are included in Hemidoras; and Tenellus, new genus, is described to include Nemadoras trimaculatus, N. -

Dendrocnide Moroides Click on Images to Enlarge
Species information Abo ut Reso urces Hom e A B C D E F G H I J K L M N O P Q R S T U V W X Y Z Dendrocnide moroides Click on images to enlarge Family Urticaceae Scientific Name Dendrocnide moroides (Wedd.) Chew Chew, W.L. (1965) The Gardens' Bulletin Singapore 21 : 204. Common name Female flowers. Copyright Barry Jago Stinging Bush; Stinger; Stinger, Gympie; Stinger, Mulberry-leaved; Gympie; Gympi Gympi; Mulberry-leaved Stinger; Gympie Stinger Stem Usually flowers and fruits as a shrub 1-3 m tall. Leaves Twigs, petioles and both the upper and lower surfaces of the leaf blade clothed in stinging hairs which inflict long-lasting pain. Stipules caducous, about 20 x 5 mm, wedged between the petiole and the twig or stem, +/- sheathing the terminal bud. Petioles long, about as long as the leaf blade and attached to the leaf blade so Female flowers. Copyright CSIRO as to be peltate. Leaf blades about 12-22 x 11-18 cm. Lateral and reticulate veins raised on the upper surface of the leaf blade and the upper surface of the leaf blade arched between the veins. Flowers Flowers small, in inflorescences up to 15 cm long, clothed in stinging hairs. Perianth about 0.75 mm long, clothed in very short hairs but free(?) of stinging hairs. Staminal filaments about 2 mm long, twisting at anthesis. Pollen white. Ovary glabrous. Fruit Infructescence up to 15 cm long consisting of a number of +/- globular heads arranged in panicles. Nuts or achenes resemble small seeds and are surrounded by the fleshy, watery, swollen receptacles or pedicels.