Synthesis, Crystal Structure and Physical Properties of Namnfe(Moo4)3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cryptochalcite K2cu5o(SO4)5
Cryptochalcite K2Cu5O(SO4)5 Crystal Data: Triclinic. Point Group: 1. As poorly formed prismatic to blocky crystals or irregular grains to 0.3 mm and in open-work aggregates to 1.2 mm. Physical Properties: Cleavage: None. Fracture: Uneven. Tenacity: Brittle. Hardness = 3 D(meas.) = n.d. D(calc.) = 3.411 Water soluble and hydroscopic. Visually not distinguishable from cesiodymite. Optical Properties: Transparent to translucent. Color: Light green to green, occasionally with a yellowish hue. Streak: Pale green. Luster: Vitreous. Optical Class: Biaxial (-). α = 1.610(3) β = 1.632(4) γ = 1.643(4) 2V(meas.) = 65(5)° 2V(calc.) = 70° Pleochroism: Distinct, Z = bright green, Y = green with a weak yellowish hue, X = pale green to almost colorless. Absorption: Z > Y > X. Cell Data: Space Group: P1. a = 10.0045(3) b = 12.6663(4) c = 14.4397(5) α = 102.194(3)° β = 101.372(3)° γ = 90.008(3)° Z = 4 X-ray Powder Pattern: Yadovitaya fumarole, Tolbachik volcano, Kamchatka Peninsula, Russia. 6.95 (100), 3.93 (65), 6.22 (45), 3.19 (35), 13.9 (30), 3.76 (30), 3.39 (30) Chemistry: (1) Na2O 0.30 K2O 9.55 Rb2O 0.89 Cs2O 0.90 MgO 0.83 CuO 33.95 ZnO 9.14 SO3 44.06 Total 99.62 (1) Yadovitaya fumarole, Second scoria cone, Tolbachik volcano, Kamchatka Peninsula, Russia; average of 4 electron microprobe analyses supplemented by Raman spectroscopy; corresponds to (K1.83Na0.09Rb0.09Cs0.06)Σ=2.07(Cu3.86Zn1.02Mg0.19)Σ=5.07S4.97O21. Polymorphism & Series: Forms a solid-solution series with cesiodymite. -

Shin-Skinner January 2018 Edition
Page 1 The Shin-Skinner News Vol 57, No 1; January 2018 Che-Hanna Rock & Mineral Club, Inc. P.O. Box 142, Sayre PA 18840-0142 PURPOSE: The club was organized in 1962 in Sayre, PA OFFICERS to assemble for the purpose of studying and collecting rock, President: Bob McGuire [email protected] mineral, fossil, and shell specimens, and to develop skills in Vice-Pres: Ted Rieth [email protected] the lapidary arts. We are members of the Eastern Acting Secretary: JoAnn McGuire [email protected] Federation of Mineralogical & Lapidary Societies (EFMLS) Treasurer & member chair: Trish Benish and the American Federation of Mineralogical Societies [email protected] (AFMS). Immed. Past Pres. Inga Wells [email protected] DUES are payable to the treasurer BY January 1st of each year. After that date membership will be terminated. Make BOARD meetings are held at 6PM on odd-numbered checks payable to Che-Hanna Rock & Mineral Club, Inc. as months unless special meetings are called by the follows: $12.00 for Family; $8.00 for Subscribing Patron; president. $8.00 for Individual and Junior members (under age 17) not BOARD MEMBERS: covered by a family membership. Bruce Benish, Jeff Benish, Mary Walter MEETINGS are held at the Sayre High School (on Lockhart APPOINTED Street) at 7:00 PM in the cafeteria, the 2nd Wednesday Programs: Ted Rieth [email protected] each month, except JUNE, JULY, AUGUST, and Publicity: Hazel Remaley 570-888-7544 DECEMBER. Those meetings and events (and any [email protected] changes) will be announced in this newsletter, with location Editor: David Dick and schedule, as well as on our website [email protected] chehannarocks.com. -

New Mineral Names*,†
American Mineralogist, Volume 98, pages 2201–2204, 2013 New Mineral Names*,† DMITRIY BELAKOVSKIY Fersman Mineralogical Museum, Russian Academy of Sciences, Moscow, Russia IN THIS ISSUE This New Mineral Names has entries for five new minerals, including christofschäferite-(Ce), kasatkinite, osumilite-(Mg), steklite, and vigrishinite. These new minerals have been published in Zapiski Rossiyskogo Mineralogicheskogo Obshchestva (Proceedings of the Russian Mineralogical Society) and in Novye dannye o mineralakh (New data on minerals). CHRISTOFSCHÄFERITE‑(CE)* chemical analyses (WDS, valency of Mn by XANES data, N.V. Chukanov, S.M. Aksenov, R.K. Rastsvetaeva, D.I. Bela- wt%) is: CaO 2.61 (2.24–2.98), La2O3 19.60 (19.20–19.83), kovskiy, J. Göttlicher, S.N. Britvin, and S. Möckel (2012) Ce2O3 22.95 (22.84–23.06), Pr2O3 0.56 (0.43–0.68), Nd2O3 2.28 2+ 3+ 3+ (2.01–2.50), MgO 0.08 (0–0.20), MnO 4.39 (4.27–4.51), total Christofschäferite-(Ce), (Ce,La,Ca)4Mn (Ti,Fe )3(Fe , 2+ Fe as FeO 6.98 (6.74–7.26) (apportioned in the proportions Fe ,Ti) (Si2O7)2O8, a new chevkinite-group mineral from 2+ 3+ the Eifel area, Germany. Novye dannye o mineralakh, 47, Fe :Fe = 3:2, by structural data: FeO 4.18, Fe2O3 3.11), Al2O3 33–42 (in English). 0.08 (0–0.19), TiO2 19.02 (18.64–19.39), Nb2O5 0.96 (0.83–1.11), SiO2 19.38 (19.16–19.52), total 99.20. The empirical formula 2+ 2+ A new mineral, christofschäferite-(Ce), was discovered at based on 22 O is (Ce1.72La1.48Nd0.17Pr0.04Ca0.57)Σ3.98Mn 0.76Fe 0.72 3+ the famous outcrop Wingertsbergwand (Wingertsberg Mt.) Mg0.02Ti2.935Fe 0.48Al0.02Nb0.09Si3.98O22. -

Curriculum Vitæ
Curriculum Vitæ JOHN M. HUGHES Address: Department of Geolgy University of Vermont 321 Delahanty Hall Burlington, VT 05405 VOX: 802-656-9443; Fax: 802-656-0045 [email protected] Education: Ph.D., Dartmouth College, 1981 Predoctoral Fellow, Geophysical Laboratory, Carnegie Institution of Washington, 1980 A.M., Dartmouth College, 1978 A.B., Franklin and Marshall College, 1975 Academic Employment History: Administrative University of Vermont: 2006 – 2009: Provost and Senior Vice President Miami University: 2003- 2006: Associate Provost for Research & Scholarship, Dean of the Graduate School 2000-2002: Associate Dean, College of Arts and Science 1999-2000: Interim Associate Dean, College of Arts and Science 1992-1999: Chair, Dept. of Geology 1987-1992: Associate Chair, Dept. of Geology Academic University of Vermont: 2006 – : Professor, Dept. of Geology Miami University: 1992-2007: Professor, Dept. of Geology 1986-1992: Associate Professor, Dept. of Geology 1981-1986: Assistant Professor, Dept. of Geology Awards, Fellowships and Honors: A Special Session was held at the Geological Society of America National Meeting “In Honor of the Career Accomplishments of John M. Hughes”. Selected as the Distinguished Speaker by C2/m Mineralogy, Inc., at the 100th Anniversary Celebration Meeting of the Mineralogical Society of America. Selected at the 2019 Honorary Award recipient of the American Federation of Mineralogical Societies. The award includes a $4,000 scholarship for a student of my selection. 2 2017 Hawley Medal recipient, Mineralogical Association of Canada (with G. Tansman, P. Kindstedt. The mineral ophirite was named 2015 Mineral of the Year by the International Mineralogical Association based on a published paper. Invited Commencement Speaker, Department of Geosciences, Virginia Tech, 2013. -

IMA Master List
The New IMA List of Minerals – A Work in Progress – Updated: March 2014 In the following pages of this document a comprehensive list of all valid mineral species is presented. The list is distributed (for terms and conditions see below) via the web site of the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association, which is the organization in charge for approval of new minerals, and more in general for all issues related to the status of mineral species. The list, which will be updated on a regular basis, is intended as the primary and official source on minerals. Explanation of column headings: Name : it is the presently accepted mineral name (and in the table, minerals are sorted by name). Chemical formula : it is the CNMNC-approved formula. IMA status : A = approved (it applies to minerals approved after the establishment of the IMA in 1958); G = grandfathered (it applies to minerals discovered before the birth of IMA, and generally considered as valid species); Rd = redefined (it applies to existing minerals which were redefined during the IMA era); Rn = renamed (it applies to existing minerals which were renamed during the IMA era); Q = questionable (it applies to poorly characterized minerals, whose validity could be doubtful). IMA No. / Year : for approved minerals the IMA No. is given: it has the form XXXX-YYY, where XXXX is the year and YYY a sequential number; for grandfathered minerals the year of the original description is given. In some cases, typically for Rd and Rn minerals, the year may be followed by s.p. -

The Synthesis and Some Properties of New Compound Cu13fe4v10o44
J Therm Anal Calorim (2012) 110:1161–1166 DOI 10.1007/s10973-011-2082-8 The synthesis and some properties of new compound Cu13Fe4V10O44 Anna Blonska-Tabero Received: 23 September 2011 / Accepted: 16 November 2011 / Published online: 4 December 2011 Ó The Author(s) 2011. This article is published with open access at Springerlink.com Abstract A new compound with the formula Cu13Fe4V10O44 forming as a result of reactions of the above vana- has been obtained as a result of a solid-state reactions dates(V) will also prove attractive from the catalytic occurring during heating the mixtures both of oxides: CuO, application point of view. Thus, the system CuO–V2O5– V2O5,Fe2O3 and vanadates: Cu5V2O10,Cu3Fe4V6O24. The Fe2O3 seems as a reasonable choice in the search for new DTA measurements were used to choose the heating tem- potential catalysts of selective oxidation of organic peratures as well as for determination of thermal stability compounds. of the new compound. Cu13Fe4V10O44 melts incongruently It is known that in one of the cross sections of the CuO– at 790 ± 5 °C. The new phase crystallizes in the mono- V2O5–Fe2O3 system, i.e. in the system FeVO4–Cu3V2O8,a clinic system. The indexing results and the calculated unit double vanadate(V) of the formula Cu3Fe4V6O24 is formed cell parameters for Cu13Fe4V10O44 are given. Its infra-red [3–5]. This compound is met in two crystallographic spectrum and image obtained by means of an electron modifications. The a-Cu3Fe4V6O24 form is a mineral called scanning microscope are presented. lyonsite [6]. The synthesis of Cu3Fe4V6O24 by the con- ventional solid-state reaction leads to its another form, i.e. -
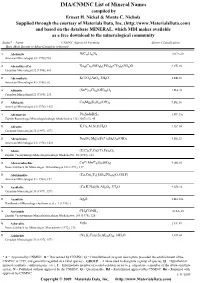
IMA/CNMNC List of Mineral Names
IMA/CNMNC List of Mineral Name s compiled by Ernest H. Nickel & Monte C. Nichols Supplied through the courtesy of Materials Data, Inc. (http://www.MaterialsData.com) and based on the database MINERAL, which MDI makes available as a free download to the mineralogical community Status* Name CNMNC Approved Formula Strunz Classification Best, Most Recent or Most Complete reference. A Abelsonite NiC£¡H£¢N¤ 10.CA.20 American Mineralogist 63 (1978) 930 A Abenakiite-(Ce) Na¢¦Ce¦(SiO£)¦(PO¤)¦(CO£)¦(SO¢)O 9.CK.10 Canadian Mineralogist 32 (1994), 843 G Abernathyite K(UO¢)AsO¤•3H¢O 8.EB.15 American Mineralogist 41 (1956), 82 A Abhurite (SnÀÈ)¢¡Cl¡¦(OH)¡¤O¦ 3.DA.30 Canadian Mineralogist 23 (1985), 233 D Abkhazite Ca¢Mg¥Si¨O¢¢(OH)¢ 9.DE.10 American Mineralogist 63 (1978), 1023 A Abramovite Pb¢SnInBiS§ 2.HF.25a Zapiski Rossiiskogo Mineralogicheskogo Obshchetstva 136 (2007) (5), 45 D Abrazite K,Ca,Al,Si,O,H¢O 9.GC.05 Canadian Mineralogist 35 (1997), 1571 D Abriachanite Na¢(Fe,Mg)£(FeÁÈ)¢Si¨O¢¢(OH)¢ 9.DE.25 American Mineralogist 63 (1978), 1023 D Absite (U,Ca,Y,Ce)(Ti,Fe)¢O¦ Zapiski Vsesoyuznogo Mineralogicheskogo Obshchestva 92 (1963), 113 A Abswurmbachite CuÀÈ(MnÁÈ)¦O¨(SiO¤) 9.AG.05 Neues Jahrbuch für Mineralogie, Abhandlungen 163 (1991), 117 D Abukumalite (Ca,Ce)¢Y£(SiO¤,PO¤)£(O,OH,F) American Mineralogist 51 (1966), 152 D Acadialite (Ca,K,Na)(Si,Al)£O¦•3H¢O 9.GD.10 Canadian Mineralogist 35 (1997), 1571 G Acanthite Ag¢S 2.BA.30a Handbook of Mineralogy (Anthony et al.), 1 (1990), 1 A Acetamide CH£CONH¢ 10.AA.20 Zapiski Vsesoyuznogo Mineralogicheskogo -

New Acquisitions to the Fersman Mineralogical Museum, Russian Academy of Sciences
New Data on Minerals. M., 2004. Vol. 39 147 UDK 549:069 NEW ACQUISITIONS TO THE FERSMAN MINERALOGICAL MUSEUM, RUSSIAN ACADEMY OF SCIENCES. 2002–2003 Dmitriy I. Belakovskiy, Fersman Mineralogical Museum, RAS, email: [email protected]; website: http://www.fmm.ru A total of 1,356 new mineral specimens were cataloged into the Fersman Mineralogical Museum main collec- tions during the period 2002 to 2003. These specimens represent 640 different mineral species from 62 coun- tries. Among these, 285 are new species for the Museum, including 10 species that were discovered by Museum staff members and 40 species that were discovered during this period by others. Of the minerals obtained, 54 are either type specimens or fragments of type specimens or cotypes. By the end of 2003 the num- ber of valid mineral species in the Museum reached 2,910. Of the newly acquired items, approximately 51% were donated by 138 people and by 8 institutions, 18% were purchased, 15% specimens were collected by the Museum staff, 12% were exchanged with collectors and other museums, 3% were acquired as type specimens and 1% obtained in other ways. A review of the new acquisitions is presented by mineral species, geography, acquisition type, and source. The review is accompanied by a list of new species for the Museum along with a list of species that the Museum desires to obtain. A total of 1356 specimens were cataloged of the Museum’s major collections in years into the Museum’s five main collections1 in the 2002 and 2003. Specimens that had not, at that years 2002–2003. -

Phases in the Subsolidus Area of the System Cuo–V2O5–Fe2o3
J Therm Anal Calorim (2012) 109:685–691 DOI 10.1007/s10973-012-2210-0 Phases in the subsolidus area of the system CuO–V2O5–Fe2O3 Anna Blonska-Tabero Bretsznajder Special Chapter Ó The Author(s) 2012. This article is published with open access at Springerlink.com Abstract The phase equilibria in the solid state in the sys- phases, very important is the knowledge of the range of tem FeVO4–Cu3V2O8 and FeVO4–CuO have been deter- their coexistence with the other compounds formed in a mined. Based on the obtained DTA and XRD analysis results given multicomponent system. and some additional research, a phase diagram in the whole Compounds forming in the lateral systems of the ternary subsolidus area of the system CuO–V2O5–Fe2O3 has been system CuO–V2O5–Fe2O3 as well as their properties are worked out. Eighteen subsidiary subsystems can be distin- known [4–6]. Recently, as a result of the research on phase guished in this ternary system. Basic properties of the obtained relations in the limited concentration range of the compo- phases with howardevansite- and lyonsite-type structure have nents of this ternary system, unknown till now compound been investigated by DTA, IR, and SEM methods. with the formula Cu13Fe4V10O44 has been obtained [7]. This compound crystallizes in the monoclinic system and melts Keywords System CuO–V2O5–Fe2O3 Á DTA Á XRD Á incongruently at 790 ± 5 °C[7]. In the system CuO–V2O5– IR Á Solid-state reactions Fe2O3 also are formed another two phases: one with howardevansite-type structure and the other with lyonsite- type structure. -

Characteristics of Cu and UV Deposits in the Paradox Basin
©2018 Society of Economic Geologists, Inc. Guidebook Series, Volume 59 Characteristics of Cu and U-V Deposits in the Paradox Basin (Colorado Plateau) and Associated Alteration Isabel F. Barton,1,2,† Mark D. Barton,2,3 Jon P. Thorson4 1 Present affiliation: Department of Mining and Geological Engineering, University of Arizona, 1235 E. James E. Rogers Way in Tucson, Arizona 85721 2 Lowell Institute for Mineral Resources, 1235 E. James E. Rogers Way Room 209, Tucson, Arizona 85721 3 Department of Geosciences, University of Arizona, Tucson, Arizona 85721 4Consulting Geologist, 3611 South Xenia Street, Denver, Colorado 80237 Introduction The complex, 300-m.y. evolution of the Paradox Basin featured numerous paleofluids of diverse types. Their flow through rocks, and interactions with each other and with the rocks, created zones of alteration and mineralization that are still visible today. Studying these provides valuable insights into the processes of paleofluid flow and rock alteration, not only in the Paradox Basin, but in basinal systems worldwide. This article presents some preliminary geological and mineralogical descriptions of altered (± mineralized) areas that we will visit on the field trip and discusses their implications for the nature, timing, and effects of paleofluid flow in the Paradox Basin. This work is part of an ongoing study of paleofluids in the Paradox Basin, so all results presented here are preliminary and are necessarily incomplete. For geologic and geochemical background, the reader is referred to the introductions by Jon Thorson (2018) and Mark Barton et al. (2018). Figure 1 provides a schematic overview of the stratigraphy and the stratigraphic distribution of some of the alteration and mineralization features we will see on the field trip. -

IMA Master List
The New IMA List of Minerals – A Work in Progress – Updated: October 2013 In the following pages of this document a comprehensive list of all valid mineral species is presented. The list is distributed (for terms and conditions see below) via the web site of the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association, which is the organization in charge for approval of new minerals, and more in general for all issues related to the status of mineral species. The list, which will be updated on a regular basis, is intended as the primary and official source on minerals. Explanation of column headings: Name: it is the presently accepted mineral name (and in the table, minerals are sorted by name). Chemical formula: it is the CNMNC-approved formula. IMA status: A = approved (it applies to minerals approved after the establishment of the IMA in 1958); G = grandfathered (it applies to minerals discovered before the birth of IMA, and generally considered as valid species); Rd = redefined (it applies to existing minerals which were redefined during the IMA era); Rn = renamed (it applies to existing minerals which were renamed during the IMA era); Q = questionable (it applies to poorly characterized minerals, whose validity could be doubtful). IMA No. / Year: for approved minerals the IMA No. is given: it has the form XXXX-YYY, where XXXX is the year and YYY a sequential number; for grandfathered minerals the year of the original description is given. In some cases, typically for Rd and Rn minerals, the year may be followed by s.p. -

Steklite Kal(SO4)2
Steklite KAl(SO4)2 Crystal Data: Hexagonal. Point Group: 32. As hexagonal or irregularly shaped crystals, platy on (001), to 1 mm. Crystals often split and combined in open aggregates or thin crusts. Physical Properties: Cleavage: Perfect on {001}. Tenacity: Brittle. Fracture: n.d. Hardness = 2.5 D(meas.) = n.d. D(calc.) = 2.797 Optical Properties: Transparent. Color: Colorless, white to grayish-white in aggregates. Streak: White. Luster: Vitreous. Optical Class: Uniaxial (-). ω = 1.546(2) ε = 1.533(3) Pleochroism: None. Cell Data: Space Group: P321. a = 4.7281(3) c = 7.9936(5) Z = 1 X-ray Powder Pattern: Tolbachik volcano, Kamchatka, Russia. 3.649 (100), 2.861 (51), 8.02 (34), 2.364 (25), 2.660 (19), 2.267 (14), 1.822 (12), 4.085 (11) Chemistry: (1) Na2O 0.09 K2O 18.12 CaO 0.08 MnO 0.03 Fe2O3 2.02 Al2O3 18.18 SO3 61.80 Total 100.37 (1) Tolbachik volcano, Kamchatka, Russia; average of 5 electron microprobe analyses, corresponds 3+ to (K0.997Na0.008Ca0.004)Σ=1.009(Al0.925 Fe 0.066Mg0.003Mn0.001)Σ=0 995S2.001O8. Occurrence: A volcanic sublimate formed at 150-170 °C as part of sulfate crusts around an active fumarole. Also described as a sublimate near burning coal deposits. Association: Alumoklyuchevskite, langbeinite, euchlorine, fedotovite, chalcocyanite, hematite, kamchatkite, atlasovite, melanothallite, tenorite, avdoninite, belloite, ziesite, Cu-lyonsite (Tolbackhik, Russia). Distribution: From the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption (1975–1976), Tolbachik volcano, Kamchatka, and the dumps of coal mine N 47 near Kopeisk, South Urals, Russia.