From Japan, with Inference on the Phylogenetic Position of Plehniidae
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Natural Products in Polyclad Flatworms
marine drugs Review Natural Products in Polyclad Flatworms Justin M. McNab 1 , Jorge Rodríguez 1, Peter Karuso 2,* and Jane E. Williamson 1,* 1 Department of Biological Sciences, Macquarie University, Sydney, NSW 2109, Australia; [email protected] (J.M.M.); [email protected] (J.R.) 2 Department of Molecular Sciences, Macquarie University, Sydney, NSW 2109, Australia * Correspondence: [email protected] (P.K.); [email protected] (J.E.W.) Abstract: Marine invertebrates are promising sources of novel bioactive secondary metabolites, and organisms like sponges, ascidians and nudibranchs are characterised by possessing potent defensive chemicals. Animals that possess chemical defences often advertise this fact with aposematic colouration that potential predators learn to avoid. One seemingly defenceless group that can present bright colouration patterns are flatworms of the order Polycladida. Although members of this group have typically been overlooked due to their solitary and benthic nature, recent studies have isolated the neurotoxin tetrodotoxin from these mesopredators. This review considers the potential of polyclads as potential sources of natural products and reviews what is known of the activity of the molecules found in these animals. Considering the ecology and diversity of polyclads, only a small number of species from both suborders of Polycladida, Acotylea and Cotylea have been investigated for natural products. As such, confirming assumptions as to which species are in any sense toxic or if the compounds they use are biosynthesised, accumulated from food or the product of symbiotic bacteria is difficult. However, further research into the group is suggested as these animals often display aposematic colouration and are known to prey on invertebrates rich in bioactive secondary metabolites. -

Chapter Two Marine Organisms
THE SINGAPORE BLUE PLAN 2018 EDITORS ZEEHAN JAAFAR DANWEI HUANG JANI THUAIBAH ISA TANZIL YAN XIANG OW NICHOLAS YAP PUBLISHED BY THE SINGAPORE INSTITUTE OF BIOLOGY OCTOBER 2018 THE SINGAPORE BLUE PLAN 2018 PUBLISHER THE SINGAPORE INSTITUTE OF BIOLOGY C/O NSSE NATIONAL INSTITUTE OF EDUCATION 1 NANYANG WALK SINGAPORE 637616 CONTACT: [email protected] ISBN: 978-981-11-9018-6 COPYRIGHT © TEXT THE SINGAPORE INSTITUTE OF BIOLOGY COPYRIGHT © PHOTOGRAPHS AND FIGURES BY ORINGAL CONTRIBUTORS AS CREDITED DATE OF PUBLICATION: OCTOBER 2018 EDITED BY: Z. JAAFAR, D. HUANG, J.T.I. TANZIL, Y.X. OW, AND N. YAP COVER DESIGN BY: ABIGAYLE NG THE SINGAPORE BLUE PLAN 2018 ACKNOWLEDGEMENTS The editorial team owes a deep gratitude to all contributors of The Singapore Blue Plan 2018 who have tirelessly volunteered their expertise and effort into this document. We are fortunate to receive the guidance and mentorship of Professor Leo Tan, Professor Chou Loke Ming, Professor Peter Ng, and Mr Francis Lim throughout the planning and preparation stages of The Blue Plan 2018. We are indebted to Dr. Serena Teo, Ms Ria Tan and Dr Neo Mei Lin who have made edits that improved the earlier drafts of this document. We are grateful to contributors of photographs: Heng Pei Yan, the Comprehensive Marine Biodiversity Survey photography team, Ria Tan, Sudhanshi Jain, Randolph Quek, Theresa Su, Oh Ren Min, Neo Mei Lin, Abraham Matthew, Rene Ong, van Heurn FC, Lim Swee Cheng, Tran Anh Duc, and Zarina Zainul. We thank The Singapore Institute of Biology for publishing and printing the The Singapore Blue Plan 2018. -

Platyhelminthes) at the Queensland Museum B.M
VOLUME 53 ME M OIRS OF THE QUEENSLAND MUSEU M BRIS B ANE 30 NOVE mb ER 2007 © Queensland Museum PO Box 3300, South Brisbane 4101, Australia Phone 06 7 3840 7555 Fax 06 7 3846 1226 Email [email protected] Website www.qm.qld.gov.au National Library of Australia card number ISSN 0079-8835 Volume 53 is complete in one part. NOTE Papers published in this volume and in all previous volumes of the Memoirs of the Queensland Museum may be reproduced for scientific research, individual study or other educational purposes. Properly acknowledged quotations may be made but queries regarding the republication of any papers should be addressed to the Editor in Chief. Copies of the journal can be purchased from the Queensland Museum Shop. A Guide to Authors is displayed at the Queensland Museum web site www.qm.qld.gov.au/organisation/publications/memoirs/guidetoauthors.pdf A Queensland Government Project Typeset at the Queensland Museum THE STUDY OF TURBELLARIANS (PLATYHELMINTHES) AT THE QUEENSLAND MUSEUM B.M. ANGUS Angus, B.M. 2007 11 30: The study of turbellarians (Platyhelminthes) at the Queensland Museum. Memoirs of the Queensland Museum 53(1): 157-185. Brisbane. ISSN 0079-8835. Turbellarian research was largely ignored in Australia, apart from some early interest at the turn of the 19th century. The modern study of this mostly free-living branch of the phylum Platyhelminthes was led by Lester R.G. Cannon of the Queensland Museum. A background to the study of turbellarians is given particularly as it relates to the efforts of Cannon on symbiotic fauna, and his encouragement of visiting specialists and students. -

Studying Early Embryogenesis in the Flatworm Maritigrella Crozieri
bioRxiv preprint doi: https://doi.org/10.1101/610733; this version posted April 18, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 2 Studying early embryogenesis in the flatworm Maritigrella 3 crozieri indicates a unique modification of the spiral cleavage 4 program in polyclad flatworms 5 6 7 8 Johannes Girstmair1,2, Maximilian J. Telford1 * 9 10 1 Centre for Life’s Origins and Evolution, Department of Genetics, Evolution and Environment, 11 University College London, London, WC1E 6BT United Kingdom 12 * corresponding author: [email protected] 13 14 2 Max Planck Institute of Molecular Cell Biology and Genetics, Pfotenhauerstraße 108, 01307 Dresden, 15 Germany 16 [email protected] 17 18 19 1 bioRxiv preprint doi: https://doi.org/10.1101/610733; this version posted April 18, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 20 Abstract 21 Background: Spiral cleavage is a conserved early developmental mode found in several 22 phyla of Lophotrochozoans with highly diverse adult body plans. While the cleavage pattern 23 has clearly been broadly conserved, it has also undergone many modifications in various taxa. 24 The precise mechanisms of how different adaptations have altered the ancestral spiral 25 cleavage pattern is an important ongoing evolutionary question and adequately answering this 26 question requires obtaining a broad developmental knowledge of different spirally cleaving 27 taxa. -

Platyhelminthes
Journal of the Marine Biological Association of the United Kingdom, page 1 of 12. # Marine Biological Association of the United Kingdom, 2014 doi:10.1017/S0025315414001106 Five new records and one new species of Polycladida (Platyhelminthes) for the Cantabrian coast (North Atlantic) of the Iberian Peninsula daniel marquina1, fernando a’ ngel ferna’ ndez-a’ lvarez1,2 and carolina noren~a1 1Department of Biodiversity and Evolutionary Biology, Museo Nacional de Ciencias Naturales (CSIC), Calle Jose´ Gutie´rrez Abascal, 2, 28006 Madrid, Spain, 2Present address: Institut de Cie`ncies del Mar (CSIC), Passeig Maritim, 37-49, E-08003 Barcelona, Spain The Iberian Peninsula is part of the South European Atlantic Shelf within the Lusitanian ecoregion. Given the characteristics of this region, a great invertebrate biodiversity is expected. Nevertheless, no literature records of Polycladida are known for the Cantabrian Sea. Here, we report the presence of six polyclad species, including one new species. Notoplana vitrea, considered endemic to the Mediterranean Sea, was found in the Cantabrian Sea, demonstrating its presence in Atlantic waters. This species was previously reported for these waters on two natural history photographic websites: the importance of searching, indexing and disseminating this type of record for the scientific community is discussed. Discocelis tigrina is reported for the first time for the Cantabrian Sea, and is the northernmost record to date. In this paper, Pleioplana atomata is reported for the second time for the Iberian Peninsula, yet is the first record for the Cantabrian Sea. Although a literature record of Leptoplana tremellaris for the Iberian Peninsula exists, it is considered a misidentification of L. -

From Cape Verde and Related Regions of Macaronesia
European Journal of Taxonomy 736: 1–43 ISSN 2118-9773 https://doi.org/10.5852/ejt.2021.736.1249 www.europeanjournaloftaxonomy.eu 2021 · Cuadrado D. et al. This work is licensed under a Creative Commons Attribution License (CC BY 4.0). Research article urn:lsid:zoobank.org:pub:FC9085BE-73C4-4F33-BD9B-6A9F573AB01D Polycladida (Platyhelminthes, Rhabditophora) from Cape Verde and related regions of Macaronesia Daniel CUADRADO 1, Jorge RODRÍGUEZ 2, Leopoldo MORO 3, Cristina GRANDE 4 & Carolina NOREÑA 5,* 1,5 Departmento de Biodiversidad y Biología Evolutiva, Museo Nacional de Ciencias Naturales (CSIC), c/ José Gutiérrez Abascal 2, 28006 Madrid, Spain. 2 Marine Invertebrates Department, Australian Museum Research Institute, Australian Museum, 1 William Street, Sydney, NSW 2010, Australia. 3 Servicio de Biodiversidad, Gobierno de Canarias, Edif. Usos Múltiples I, Av. Anaga n° 35, Pl. 11, 38071 S/C de Tenerife, Canary Islands, Spain. 4 Departamento de Biología, Facultad de Ciencias, Universidad Autónoma de Madrid, Cantoblanco, 28049 Madrid, Spain. * Corresponding author: [email protected] 1 Email: [email protected] 2 Email: [email protected] 3 Email: [email protected] 4 Email: [email protected] 1 urn:lsid:zoobank.org:author:F0C14D94-9996-4A20-9D56-B02DDA1A78CA 2 urn:lsid:zoobank.org:author:B833502E-CBA4-40CA-AE5A-BAD02F539062 3 urn:lsid:zoobank.org:author:B66DDDE6-98E6-42FD-8E58-A1DF6A386BE5 4 urn:lsid:zoobank.org:author:C8634A50-D3EC-467A-A868-225C231B40F2 5 urn:lsid:zoobank.org:author:DD03B71F-B45E-402B-BA32-BB30343E0D95 Abstract. The systematics and distribution of the order Polycladida within the Macaronesian archipelagos are analysed. New species (Marcusia alba sp. -
Platyhelminthes, Polycladida)
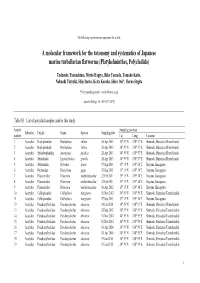
The following supplement accompanies the article A molecular framework for the taxonomy and systematics of Japanese marine turbellarian flatworms (Platyhelminthes, Polycladida) Tadasuke Tsunashima, Morio Hagiya, Riko Yamada, Tomoko Koito, Nobuaki Tsuyuki, Shin Izawa, Keita Kosoba, Shiro Itoi*, Haruo Sugita *Corresponding author: [email protected] Aquatic Biology 26: 159–167 (2017) Table S1. List of polyclad samples used in this study Sample Sampling location Suborder Family Genus Species Sampling date number Lat. Long. Location 1 Acotylea Hoploplanidae Hoploplana villosa 26 Apr 2013 34° 39' N 138° 57' E Shimoda, Shizuoka (Ebisu Island) 2 Acotylea Hoploplanidae Hoploplana villosa 26 Apr 2013 34° 39' N 138° 57' E Shimoda, Shizuoka (Ebisu Island) 3 Acotylea Stylochoplanidae Amemiyaia pacifica 26 Apr 2013 34° 39' N 138° 57' E Shimoda, Shizuoka (Ebisu Island) 4 Acotylea Stylochidae Leptostylochus gracilis 26 Apr 2013 34° 39' N 138° 57' E Shimoda, Shizuoka (Ebisu Island) 5 Acotylea Stylochidae Stylochus ijimai 07 Sep 2013 35° 15' N 139° 34' E Hayama, Kanagawa 6 Acotylea Ilyplanidae Discoplana gigas 07 Sep 2013 35° 15' N 139° 34' E Hayama, Kanagawa 7 Acotylea Planoceridae Planocera multitentaculata 22 Feb 2011 35° 15' N 139° 34' E Hayama, Kanagawa 8 Acotylea Planoceridae Planocera multitentaculata 22 Feb 2011 35° 15' N 139° 34' E Hayama, Kanagawa 9 Acotylea Planoceridae Planocera multitentaculata 06 Apr 2012 35° 15' N 139° 34' E Hayama, Kanagawa 10 Acotylea Callioplanidae Callioplana marginata 01 Nov 2013 34° 39' N 138° 59' E Shimoda, Shizuoka -

An Annotated Checklist of the Marine Macroinvertebrates of Alaska David T
NOAA Professional Paper NMFS 19 An annotated checklist of the marine macroinvertebrates of Alaska David T. Drumm • Katherine P. Maslenikov Robert Van Syoc • James W. Orr • Robert R. Lauth Duane E. Stevenson • Theodore W. Pietsch November 2016 U.S. Department of Commerce NOAA Professional Penny Pritzker Secretary of Commerce National Oceanic Papers NMFS and Atmospheric Administration Kathryn D. Sullivan Scientific Editor* Administrator Richard Langton National Marine National Marine Fisheries Service Fisheries Service Northeast Fisheries Science Center Maine Field Station Eileen Sobeck 17 Godfrey Drive, Suite 1 Assistant Administrator Orono, Maine 04473 for Fisheries Associate Editor Kathryn Dennis National Marine Fisheries Service Office of Science and Technology Economics and Social Analysis Division 1845 Wasp Blvd., Bldg. 178 Honolulu, Hawaii 96818 Managing Editor Shelley Arenas National Marine Fisheries Service Scientific Publications Office 7600 Sand Point Way NE Seattle, Washington 98115 Editorial Committee Ann C. Matarese National Marine Fisheries Service James W. Orr National Marine Fisheries Service The NOAA Professional Paper NMFS (ISSN 1931-4590) series is pub- lished by the Scientific Publications Of- *Bruce Mundy (PIFSC) was Scientific Editor during the fice, National Marine Fisheries Service, scientific editing and preparation of this report. NOAA, 7600 Sand Point Way NE, Seattle, WA 98115. The Secretary of Commerce has The NOAA Professional Paper NMFS series carries peer-reviewed, lengthy original determined that the publication of research reports, taxonomic keys, species synopses, flora and fauna studies, and data- this series is necessary in the transac- intensive reports on investigations in fishery science, engineering, and economics. tion of the public business required by law of this Department. -

A New Species of Phaenoplana (Platyhelminthes: Polycladida) from the Ogasawara Islands
Species Diversity 24: 1–6 Published online 25 January 2019 DOI: 10.12782/specdiv.24.1 A New Species of Phaenoplana (Platyhelminthes: Polycladida) from the Ogasawara Islands Yuki Oya1,3 and Hiroshi Kajihara2 1 Graduate School of Science, Hokkaido University, Sapporo, Hokkaido 060-0810, Japan E-mail: [email protected] 2 Faculty of Science, Hokkaido University, Sapporo, Hokkaido 060-0810, Japan 3 Corresponding author (Received 26 July 2018; Accepted 8 November 2018) http://zoobank.org/8282F788-F3C7-4918-99D3-6C672BA8DE58 We describe a new species of polyclad flatworm, Phaenoplana kopepe sp. nov., from Chichijima island in the Ogas- awara Islands, Japan. This is the first report of Phaenoplana from Japan. Phaenoplana kopepe sp. nov. is characterized by i) a vagina that curves anteriorly, ii) gonopores well-separated from each other, and iii) a Lang’s duct that is shorter than the vagina. We provide a partial sequence (610 bp) from the mitochondrial cytochrome c oxidase subunit I gene as a DNA bar- code for the species. Key Words: Acotylea, COI, cox1, DNA barcoding, marine flatworm, Pacific, Stylochoplanidae. cus, 1952 in the reproductive organs and its epithelial ultra- Introduction structure. The morphological heterogeneity and the conflict in taxonomic opinions between Faubel (1983) and Prudhoe The polyclad flatworm genus Phaenoplana Faubel, 1983, (1985) indicate that Phaenoplana requires a thorough revi- belonging to Stylochoplanidae, is distinguished from other sion. However, such a taxonomic revision should involve ex- stylochoplanid genera by possessing a penis rod and a Lang’s tensive molecular phylogeny of acotyleans, which is beyond vesicle. Faubel (1983) assigned five species to Phaenoplana; the scope of this paper. -

Platyhelminthes: Acotylea) in the Ryukyu Islands, Japan
marine drugs Article TTX-Bearing Planocerid Flatworm (Platyhelminthes: Acotylea) in the Ryukyu Islands, Japan Hiroyuki Ueda, Shiro Itoi * and Haruo Sugita Department of Marine Science and Resources, Nihon University, Fujisawa, Kanagawa 252-0880, Japan; [email protected] (H.U.); [email protected] (H.S.) * Correspondence: [email protected]; Tel./Fax: +81-466-84-3679 Received: 10 December 2017; Accepted: 17 January 2018; Published: 19 January 2018 Abstract: Polyclad flatworms comprise a highly diverse and cosmopolitan group of marine turbellarians. Although some species of the genera Planocera and Stylochoplana are known to be tetrodotoxin (TTX)-bearing, there are few new reports. In this study, planocerid-like flatworm specimens were found in the sea bottom off the waters around the Ryukyu Islands, Japan. The bodies were translucent with brown reticulate mottle, contained two conical tentacles with eye spots clustered at the base, and had a slightly frilled-body margin. Each specimen was subjected to TTX extraction followed by liquid chromatography with tandem mass spectrometry analysis. Mass chromatograms were found to be identical to those of the TTX standards. The TTX amounts in the two flatworm specimens were calculated to be 468 and 3634 µg. Their external morphology was found to be identical to that of Planocera heda. Phylogenetic analysis based on the sequences of the 28S rRNA gene and cytochrome-c oxidase subunit I gene also showed that both specimens clustered with the flatworms of the genus Planocera (Planocera multitentaculata and Planocera reticulata). This fact suggests that there might be other Planocera species that also possess highly concentrated TTX, contributing to the toxification of TTX-bearing organisms, including fish. -

Newman Et Al 2003
Micronesica 35-36:189-199. 2003 Checklist of polyclad flatworms (Platyhelminthes) from Micronesian coral reefs L. J. NEWMAN School of Environmental Science & Management Southern Cross University PO Box 157 Lismore, NSW Australia 2480 email:[email protected] G. PAULAY1, R. RITSON-WILLIAMS2 Marine Laboratory University of Guam Mangilao, Guam 96923 U.S.A AbstractWe record 68 species of polyclad flatworms from new material (all photo-documented) and 28 species from literature records, for a total diversity of 88 species for Micronesia. Up to 60% of the encountered species may be undescribed. Guam has the largest recorded fauna with 59 species, followed by 28 species known from Palau. Pseudocerotidae comprise 58% of documented species, and more than 3 times as many cotyleans than acotyleans are documented. This study shows that the polyclad fauna of Micronesia is diverse yet poorly known, and highlights the need for further work. Introduction Polyclad flatworms are conspicuous inhabitants of coral reefs especially throughout the Indo-West Pacific, yet their diversity within Micronesia remains poorly documented. Only five papers have dealt with the polyclad fauna of this large and diverse area (Kato 1943, Hyman 1955, 1959; Newman & Cannon 1997, Newman & Schupp 2002). This checklist represents the first comprehensive account of polyclad flatworms from Micronesian waters. Although many tropical polyclads are brightly colored and attract attention, they remain understudied partly for methodological reasons. Accurate taxonomic determinations involve examination of both the morphology of living animals and the anatomy of the reproductive structures (Newman & Cannon 1994a). Diagnostic color characters tend to disappear rapidly after fixation and need to be documented photographically from living animals. -

STUDIES on the FAUNA of CURAÇAO and OTHER CARIBBEAN ISLANDS: No
STUDIES ON THE FAUNA OF CURAÇAO AND OTHER CARIBBEAN ISLANDS: No. 101. Polycladida from Curaçao and faunistically related regions by Eveline du Bois-Reymond Marcus & Ernst Marcus (Departamento de Zoologia, Universidade de Sao Paulo) Professor Dr. DIVA DINIZ CORRÊA, Head of the Department of Zoology of the University of São Paulo, was able to work at the “Caraïbisch Marien-Biologisch Instituut” (Caribbean Marine Bio- logical Institute: Carmabi) at Curaçao from December 1965 to March 1966, thanks to a grant received from the Government of the Netherlands. There she collected 26 species of Polyclads, and took notes of their shapes and colours. Furthermore Dr. PIETER WAGENAAR HUMMELINCK, of Utrecht, Caribbean sent us a large collection of polyclads from the area, from He had also collected in gathered on his trips 1930 to 1964. 1963 in the Miami area. We received some samples from the latter area from Prof. Dr. CORRÊA and Prof. Dr. FREDERICK M. BAYER, of Miami. Drs. LILIANA FORNERIS, WALTER NARCHI, and SÉRGIO DE ALMEIDA all of São Brazilian RODRIGUES, Paulo, gave us interesting material. The indications (B), (C), (F), (H), or (N) after the date denote the collectors. Dr. HUMMELINCK'S station numbers (H 1008A, 1064b, under of the be etc., cf. Fig. 100), which a description habitat can found, refer to his list of 1930-1949 localities in the 4th volume of this series, or to a forthcoming paper, in which the 1955-1964 lo- calities will be described. The specimens collected by Dr. HUMMELINCK and Dr. BAYER will be returned to Utrecht and Miami, respectively, the other ones are kept in the Department of Zoology, Faculty of Philosophy, University of Sao Paulo.