Axonal Action-Potential Initiation and Na Channel Densities in the Soma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
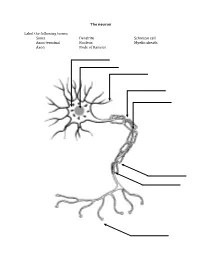
The Neuron Label the Following Terms: Soma Axon Terminal Axon Dendrite
The neuron Label the following terms: Soma Dendrite Schwaan cell Axon terminal Nucleus Myelin sheath Axon Node of Ranvier Neuron Vocabulary You must know the definitions of these terms 1. Synaptic Cleft 2. Neuron 3. Impulse 4. Sensory Neuron 5. Motor Neuron 6. Interneuron 7. Body (Soma) 8. Dendrite 9. Axon 10. Action Potential 11. Myelin Sheath (Myelin) 12. Afferent Neuron 13. Threshold 14. Neurotransmitter 15. Efferent Neurons 16. Axon Terminal 17. Stimulus 18. Refractory Period 19. Schwann 20. Nodes of Ranvier 21. Acetylcholine STEPS IN THE ACTION POTENTIAL 1. The presynaptic neuron sends neurotransmitters to postsynaptic neuron. 2. Neurotransmitters bind to receptors on the postsynaptic cell. - This action will either excite or inhibit the postsynaptic cell. - The soma becomes more positive. 3. The positive charge reaches the axon hillock. - Once the threshold of excitation is reached the neuron will fire an action potential. 4. Na+ channels open and Na+ is forced into the cell by the concentration gradient and the electrical gradient. - The neuron begins to depolarize. 5. The K+ channels open and K+ is forced out of the cell by the concentration gradient and the electrical gradient. - The neuron is depolarized. 6. The Na+ channels close at the peak of the action potential. - The neuron starts to repolarize. 7. The K+ channels close, but they close slowly and K+ leaks out. 8. The terminal buttons release neurotransmitter to the postsynaptic neuron. 9. The resting potential is overshot and the neuron falls to a -90mV (hyperpolarizes). - The neuron continues to repolarize. 10. The neuron returns to resting potential. The Synapse Label the following terms: Pre-synaptic membrane Neurotransmitters Post-synaptic membrane Synaptic cleft Vesicle Post-synaptic receptors . -

The Creation of Neuroscience
The Creation of Neuroscience The Society for Neuroscience and the Quest for Disciplinary Unity 1969-1995 Introduction rom the molecular biology of a single neuron to the breathtakingly complex circuitry of the entire human nervous system, our understanding of the brain and how it works has undergone radical F changes over the past century. These advances have brought us tantalizingly closer to genu- inely mechanistic and scientifically rigorous explanations of how the brain’s roughly 100 billion neurons, interacting through trillions of synaptic connections, function both as single units and as larger ensem- bles. The professional field of neuroscience, in keeping pace with these important scientific develop- ments, has dramatically reshaped the organization of biological sciences across the globe over the last 50 years. Much like physics during its dominant era in the 1950s and 1960s, neuroscience has become the leading scientific discipline with regard to funding, numbers of scientists, and numbers of trainees. Furthermore, neuroscience as fact, explanation, and myth has just as dramatically redrawn our cultural landscape and redefined how Western popular culture understands who we are as individuals. In the 1950s, especially in the United States, Freud and his successors stood at the center of all cultural expla- nations for psychological suffering. In the new millennium, we perceive such suffering as erupting no longer from a repressed unconscious but, instead, from a pathophysiology rooted in and caused by brain abnormalities and dysfunctions. Indeed, the normal as well as the pathological have become thoroughly neurobiological in the last several decades. In the process, entirely new vistas have opened up in fields ranging from neuroeconomics and neurophilosophy to consumer products, as exemplified by an entire line of soft drinks advertised as offering “neuro” benefits. -

Crayfish Escape Behavior and Central Synapses
Crayfish Escape Behavior and Central Synapses. III. Electrical Junctions and Dendrite Spikes in Fast Flexor Motoneurons ROBERT S. ZUCKER Department of Biological Sciences and Program in the Neurological Sciences, Stanford University, Stanford, California 94305 THE LATERAL giant fiber is the decision fiber and fast flexor motoneurons are located in for a behavioral response in crayfish in- the dorsal neuropil of each abdominal gan- volving rapid tail flexion in response to glion. Dye injections and anatomical recon- abdominal tactile stimulation (27). Each structions of ‘identified motoneurons reveal lateral giant impulse is followed by a rapid that only those motoneurons which have tail flexion or tail flip, and when the giant dentritic branches in contact with a giant fiber does not fire in response to such stim- fiber are activated by that giant fiber (12, uli, the movement does not occur. 21). All of tl ie fast flexor motoneurons are The afferent limb of the reflex exciting excited by the ipsilateral giant; all but F4, the lateral giant has been described (28), F5 and F7 are also excited by the contra- and the habituation of the behavior has latkral giant (21 a). been explained in terms of the properties The excitation of these motoneurons, of this part of the neural circuit (29). seen as depolarizing potentials in their The properties of the neuromuscular somata, does not always reach threshold for junctions between the fast flexor motoneu- generating a spike which propagates out the rons and the phasic flexor muscles have also axon to the periphery (14). Indeed, only t,he been described (13). -

Plp-Positive Progenitor Cells Give Rise to Bergmann Glia in the Cerebellum
Citation: Cell Death and Disease (2013) 4, e546; doi:10.1038/cddis.2013.74 OPEN & 2013 Macmillan Publishers Limited All rights reserved 2041-4889/13 www.nature.com/cddis Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum S-H Chung1, F Guo2, P Jiang1, DE Pleasure2,3 and W Deng*,1,3,4 NG2 (nerve/glial antigen2)-expressing cells represent the largest population of postnatal progenitors in the central nervous system and have been classified as oligodendroglial progenitor cells, but the fate and function of these cells remain incompletely characterized. Previous studies have focused on characterizing these progenitors in the postnatal and adult subventricular zone and on analyzing the cellular and physiological properties of these cells in white and gray matter regions in the forebrain. In the present study, we examine the types of neural progeny generated by NG2 progenitors in the cerebellum by employing genetic fate mapping techniques using inducible Cre–Lox systems in vivo with two different mouse lines, the Plp-Cre-ERT2/Rosa26-EYFP and Olig2-Cre-ERT2/Rosa26-EYFP double-transgenic mice. Our data indicate that Olig2/Plp-positive NG2 cells display multipotential properties, primarily give rise to oligodendroglia but, surprisingly, also generate Bergmann glia, which are specialized glial cells in the cerebellum. The NG2 þ cells also give rise to astrocytes, but not neurons. In addition, we show that glutamate signaling is involved in distinct NG2 þ cell-fate/differentiation pathways and plays a role in the normal development of Bergmann glia. We also show an increase of cerebellar oligodendroglial lineage cells in response to hypoxic–ischemic injury, but the ability of NG2 þ cells to give rise to Bergmann glia and astrocytes remains unchanged. -

Oligodendrocytes in Development, Myelin Generation and Beyond
cells Review Oligodendrocytes in Development, Myelin Generation and Beyond Sarah Kuhn y, Laura Gritti y, Daniel Crooks and Yvonne Dombrowski * Wellcome-Wolfson Institute for Experimental Medicine, Queen’s University Belfast, Belfast BT9 7BL, UK; [email protected] (S.K.); [email protected] (L.G.); [email protected] (D.C.) * Correspondence: [email protected]; Tel.: +0044-28-9097-6127 These authors contributed equally. y Received: 15 October 2019; Accepted: 7 November 2019; Published: 12 November 2019 Abstract: Oligodendrocytes are the myelinating cells of the central nervous system (CNS) that are generated from oligodendrocyte progenitor cells (OPC). OPC are distributed throughout the CNS and represent a pool of migratory and proliferative adult progenitor cells that can differentiate into oligodendrocytes. The central function of oligodendrocytes is to generate myelin, which is an extended membrane from the cell that wraps tightly around axons. Due to this energy consuming process and the associated high metabolic turnover oligodendrocytes are vulnerable to cytotoxic and excitotoxic factors. Oligodendrocyte pathology is therefore evident in a range of disorders including multiple sclerosis, schizophrenia and Alzheimer’s disease. Deceased oligodendrocytes can be replenished from the adult OPC pool and lost myelin can be regenerated during remyelination, which can prevent axonal degeneration and can restore function. Cell population studies have recently identified novel immunomodulatory functions of oligodendrocytes, the implications of which, e.g., for diseases with primary oligodendrocyte pathology, are not yet clear. Here, we review the journey of oligodendrocytes from the embryonic stage to their role in homeostasis and their fate in disease. We will also discuss the most common models used to study oligodendrocytes and describe newly discovered functions of oligodendrocytes. -

Neuregulin 1–Erbb2 Signaling Is Required for the Establishment of Radial Glia and Their Transformation Into Astrocytes in Cerebral Cortex
Neuregulin 1–erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex Ralf S. Schmid*, Barbara McGrath*, Bridget E. Berechid†, Becky Boyles*, Mark Marchionni‡, Nenad Sˇ estan†, and Eva S. Anton*§ *University of North Carolina Neuroscience Center and Department of Cell and Molecular Physiology, University of North Carolina School of Medicine, Chapel Hill, NC 27599; †Department of Neurobiology, Yale University School of Medicine, New Haven, CT 06510; and ‡CeNes Pharamceuticals, Inc., Norwood, MA 02062 Communicated by Pasko Rakic, Yale University School of Medicine, New Haven, CT, January 27, 2003 (received for review December 12, 2002) Radial glial cells and astrocytes function to support the construction mine whether NRG-1-mediated signaling is involved in radial and maintenance, respectively, of the cerebral cortex. However, the glial cell development and differentiation in the cerebral cortex. mechanisms that determine how radial glial cells are established, We show that NRG-1 signaling, involving erbB2, may act in maintained, and transformed into astrocytes in the cerebral cortex are concert with Notch signaling to exert a critical influence in the not well understood. Here, we show that neuregulin-1 (NRG-1) exerts establishment, maintenance, and appropriate transformation of a critical role in the establishment of radial glial cells. Radial glial cell radial glial cells in cerebral cortex. generation is significantly impaired in NRG mutants, and this defect can be rescued by exogenous NRG-1. Down-regulation of expression Materials and Methods and activity of erbB2, a member of the NRG-1 receptor complex, leads Clonal Analysis to Study NRG’s Role in the Initial Establishment of to the transformation of radial glial cells into astrocytes. -

Polyribosomes Associated with Synaptic Specializations on Axon Initial Segments: Localization of Protein-Synthetic Machinery at Inhibitory Synapses
The Journal of Neuroscience October 1986, 6(10): 3079-3085 Polyribosomes Associated with Synaptic Specializations on Axon Initial Segments: Localization of Protein-Synthetic Machinery at Inhibitory Synapses Oswald Steward* and Charles E. Ribak-f *Departments of Neurosurgery and Physiology, University of Virginia School of Medicine, Charlottesville, Virginia 22908, and TDepartment of Anatomy and Neurobiology, University of California at Irvine, Irvine, California 92717 Previous studies have revealed a selective association between report that showed some axonal ribosomes grouped at points polyribosomes and axospinous synapses in a variety of brain of branching and beneath synaptic contacts (Jones and Powell, regions. The present study evaluates whether polyribosomes are 1969). Therefore, they were usually considered, only in passing, also associated with the symmetrical and presumably inhibitory as exceptions to the rule. Thus, it has come to be accepted as a synaptic connections found on the initial segment of axons of central tenet that essentially all of the protein constituents of some neurons in the CNS. The initial segments of pyramidal the neuron, including those required for synaptic specializations, neurons in the sensorimotor cortex of the monkey and of granule were synthesized in the cell body, and somehow selectively cells in the hippocampus of the rat were examined. The initial transported to the sites at which they would be assembled (for segments of these cell types are contacted by GABAergic ter- a review, see Grafstein and Forman, 1980). minals that form symmetrical synaptic connections. In the pres- Over the past few years, a series of observations has given ent study, these initial segments were found to contain polyri- rise to an hypothesis that challenges the prevailing view that bosomes that tended to be selectively localized beneath the virtually all neuronal proteins are produced in the cell body and synaptic specializations. -

Nernst Potentials and Membrane Potential Changes
UNDERSTANDING MEMBRANE POTENTIAL CHANGES IN TERMS OF NERNST POTENTIALS: For seeing how a change in conductance to ions affects the membrane potential, follow these steps: 1. Make a graph with membrane potential on the vertical axis (-100 to +55) and time on the horizontal axis. 2. Draw dashed lines indicating the standard Nernst potential (equilibrium potential) for each ion: Na+ = +55 mV, K+ = -90mV, Cl- = -65 mV. 3. Draw lines below the horizontal axis showing the increased conductance to individual ions. 4. Start plotting the membrane potential on the left. Most graphs will start at resting potential (-70 mV) 5. When current injection (Stim) is present, move the membrane potential upward to Firing threshold. 6. For the time during which membrane conductance to a particular ion increases, move the membrane potential toward the Nernst potential for that ion. 7. During the time when conductance to a particular ion decreases, move the membrane potential away from the Nernst potential of that ion, toward a position which averages the conductances of the other ions. 8. When conductances return to their original value, membrane potential will go to its starting value. +55mV ACTION POTENTIAL SYNAPTIC POTENTIALS 0mV Membrane potential Firing threshold Firing threshold -65mV -90mV Time Time Na+ K+ Conductances Stim CHANGES IN MEMBRANE POTENTIAL ALLOW NEURONS TO COMMUNICATE The membrane potential of a neuron can be measured with an intracellular electrode. This 1 provides a measurement of the voltage difference between the inside of the cell and the outside. When there is no external input, the membrane potential will usually remain at a value called the resting potential. -

Rapid and Efficient Generation of Oligodendrocytes from Human
Rapid and efficient generation of oligodendrocytes PNAS PLUS from human induced pluripotent stem cells using transcription factors Marc Ehrlicha,b, Sabah Mozafaric,d,e,f, Michael Glatzab, Laura Starosta,b, Sergiy Velychkob, Anna-Lena Hallmanna,b, Qiao-Ling Cuig, Axel Schambachh, Kee-Pyo Kimb, Corinne Bachelinc,d,e,f, Antoine Marteync,d,e,f, Gunnar Hargusa,b, Radia Marie Johnsoni, Jack Antelg, Jared Sterneckertj, Holm Zaehresb,k, Hans R. Schölerb,l, Anne Baron-Van Evercoorenc,d,e,f, and Tanja Kuhlmanna,1 aInstitute of Neuropathology, University Hospital Münster, 48149 Muenster, Germany; bDepartment of Cell and Developmental Biology, Max Planck Institute for Molecular Biomedicine, 48149 Muenster, Germany; cINSERM, U1127, F-75013 Paris, France; dCNRS, UMR 7225, F-75013 Paris, France; eSorbonne Universités, Université Pierre et Marie Curie Paris 06, UM-75, F-75005 Paris, France; fInstitut du Cerveau et de la Moelle epinière-Groupe Hospitalier Pitié-Salpêtrière, F-75013 Paris, France; gMontreal Neurological Institute, McGill University, Montreal, QC, Canada H3A 2B4; hInstitute of Experimental Hematology, Hannover Medical School, 30625 Hannover, Germany; iDepartment of Physiology, McGill University, Montreal, QC, Canada H3A 2B4; jDFG Research Center for Regenerative Therapies, Technische Universität Dresden, 01307 Dresden, Germany; kMedical Faculty, Department of Anatomy and Molecular Embryology, Ruhr-University Bochum, 44801 Bochum, Germany; and lMedicial Faculty, Westphalian Wilhelms-University of Muenster, 48149 Muenster, Germany Edited by Brigid L. M. Hogan, Duke University Medical Center, Durham, NC, and approved February 1, 2017 (received for review August 30, 2016) Rapid and efficient protocols to generate oligodendrocytes (OL) more, these protocols require long culture periods (70–150 d) to + from human induced pluripotent stem cells (iPSC) are currently obtain O4 OL and show limited efficiency (9–12). -

Neurotransmitter Release Is Blocked Intracellularly by Botulinum
Proc. Nati. Acad. Sci. USA Vol. 85, pp. 4090-4094, June 1988 Neurobiology Neurotransmitter release is blocked intracellularly by botulinum neurotoxin, and this requires uptake of both toxin polypeptides by a process mediated by the larger chain (cholinergic synapse/noncholinergic synapse/intraceliular in ection/quantal release/Aplysia cahifornica) B. POULAIN*, L. TAUC*, E. A. MAISEYt, J. D. F. WADSWORTHt, P. M. MOHANt, AND J. 0. DOLLYt *Laboratoire de Neurobiologie Cellulaire et Moldculaire, Centre National de la Recherche Scientifique, Gif-sur-Yvette, 91190 France; tDepartment of Biochemistry, Imperial College, London SW7 2AZ, United Kingdom; and tDepartment of Zoology, S.V. University, Tirupati, 515502, A.P. India Communicated by C. Ladd Prosser, February 17, 1988 ABSTRACT Botulinum neurotoxins (types A and B), lacking for a multistep process of intoxication (2, 9) and, in which are microbial proteins consisting of two disulfide-linked particular, for the inactivation by BoNT of an intracellular chains, inhibit specifically and with high potency the release of component that is involved in the release of AcCho and, acetylcholine from peripheral nerve terminals. As a prerequi- perhaps, all transmitters. Furthermore, the role of the HC site for a long-term development of effective treatments for and LC of BoNT in such a scheme for its neuroparalytic botulism, the internalization and inhibitory action of the toxin action remains to be established. Pertinent questions to be and its constituent chains were examined by electrophysiolog- answered include (i) whether the HC, by virtue of its ability ical methods at identified synapses inAplysia preparations that to recognize neural membrane receptors (8, 10), allows allow both intracellular and bath application of the neurotox- functional recognition ofcholinergic terminals and whether it ins. -

Neuron Morphology Influences Axon Initial Segment Plasticity
Dartmouth College Dartmouth Digital Commons Dartmouth Scholarship Faculty Work 1-2016 Neuron Morphology Influences Axon Initial Segment Plasticity Allan T. Gulledge Dartmouth College, [email protected] Jaime J. Bravo Dartmouth College Follow this and additional works at: https://digitalcommons.dartmouth.edu/facoa Part of the Computational Neuroscience Commons, Molecular and Cellular Neuroscience Commons, and the Other Neuroscience and Neurobiology Commons Dartmouth Digital Commons Citation Gulledge, A.T. and Bravo, J.J. (2016) Neuron morphology influences axon initial segment plasticity, eNeuro doi:10.1523/ENEURO.0085-15.2016. This Article is brought to you for free and open access by the Faculty Work at Dartmouth Digital Commons. It has been accepted for inclusion in Dartmouth Scholarship by an authorized administrator of Dartmouth Digital Commons. For more information, please contact [email protected]. New Research Neuronal Excitability Neuron Morphology Influences Axon Initial Segment Plasticity1,2,3 Allan T. Gulledge1 and Jaime J. Bravo2 DOI:http://dx.doi.org/10.1523/ENEURO.0085-15.2016 1Department of Physiology and Neurobiology, Geisel School of Medicine at Dartmouth, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire 03756, and 2Thayer School of Engineering at Dartmouth, Hanover, New Hampshire 03755 Visual Abstract In most vertebrate neurons, action potentials are initiated in the axon initial segment (AIS), a specialized region of the axon containing a high density of voltage-gated sodium and potassium channels. It has recently been proposed that neurons use plasticity of AIS length and/or location to regulate their intrinsic excitability. Here we quantify the impact of neuron morphology on AIS plasticity using computational models of simplified and realistic somatodendritic morphologies. -

Presynaptic Inhibition and Antidromic Spikes in Primary Afferents of the Crayfish: a Computational and Experimental Analysis
The Journal of Neuroscience, February 1, 2001, 21(3):1007–1021 Presynaptic Inhibition and Antidromic Spikes in Primary Afferents of the Crayfish: A Computational and Experimental Analysis Daniel Cattaert,1 Fre´de´ ric Libersat,2 and Abdeljabbar El Manira3 1Laboratoire Neurobiologie et Mouvements, Centre National de la Recherche Scientifique, 13402 Marseille Cedex 20, France, 2Department of Life Sciences and Zlotowski Center for Neuroscience, Ben Gurion University of the Negev, Beer Sheva, 84105, Israel, and 3The Nobel Institute for Neurophysiology, Department of Neuroscience, Karolinska Institutet, S-171 77 Stockholm, Sweden Primary afferent depolarizations (PADs) are associated with pre- large increase in chloride conductance (up to 300 nS) is needed to synaptic inhibition and antidromic discharges in both vertebrates significantly reduce the amplitude of sensory spikes. The role of and invertebrates. In the present study, we have elaborated a the spatial organization of GABA synapses in the sensory ar- realistic compartment model of a primary afferent from the coxo- borization was analyzed, demonstrating that the most effective basipodite chordotonal organ of the crayfish based on anatomical location for GABA synapses is in the area of transition from active and electrophysiological data. The model was used to test the to passive conduction. This transition is likely to occur on the main validity of shunting and sodium channel inactivation hypotheses to branch a few hundred micrometers distal to the first branching account for presynaptic inhibition. Previous studies had demon- point. As a result of this spatial organization, antidromic spikes strated that GABA activates chloride channels located on the main generated by large-amplitude PADs are prevented from propagat- branch close to the first branching point.