Antiradical Scavenging Activity and Polyphenolic Compounds Extracted from Thai Mango Seed Kernels
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of the Import Prohibited Plants
List of the Import Prohibited Plants The Annexed Table 2 of the amended Enforcement Ordinance of the Plant Protection Law (Amended portions are under lined) Districts Prohibited Plants Quarantine Pests 1. Yemen, Israel, Saudi Arabia, Fresh fruits of akee, avocado, star berry, Mediterranean fruit fly Syria, Turkey, Jordan, Lebanon, allspice, olive, cashew nut, kiwi fruit, Thevetia (Ceratitis capitata) Albania, Italy, United Kingdom peruviana, carambola, pomegranate, jaboticaba, (Great Britain and Northern broad bean, alexandrian laurel, date palm, Ireland, hereinafter referred to as Muntingia calabura, feijoa, pawpaw, mammee "United Kingdom"), Austria, apple, longan, litchi, and plants of the genera Netherlands, Cyprus, Greece, Ficus, Phaseolus, Diospyros(excluding those Croatia, Kosovo, Switzerland, listed in appendix 41), Carissa, Juglans, Morus, Spain, Slovenia, Serbia, Germany, Coccoloba, Coffea, Ribes, Vaccinium, Hungary, France, Belgium, Passiflora, Dovyalis, Ziziphus, Spondias, Musa Bosnia and Herzegovina, (excluding immature banana), Carica (excluding Portugal, Former Yugoslav those listed in appendix 1), Psidium, Artocarpus, Republic of Macedonia, Malta, , Annona, Malpighia, Santalum, Garcinia, Vitis Montenegro, Africa, Bermuda, (excluding those listed in appendices 3 and 54), Argentina, Uruguay, Ecuador, El Eugenia, Mangifera (excluding those listed in Salvador, Guatemala, Costa Rica, appendices 2 ,36 ,43 ,51 and 53), Ilex, Colombia, Nicaragua, West Indies Terminalia and Gossypium, and Plants of the (excluding Cuba, Dominican family Sapotaceae, Cucurbitaceae (excluding Republic,Puerto Rico), Panama, those listed in appendices 3 and 42), Cactaceae Paraguay, Brazil, Venezuela, (excluding those listed in appendix 35), Peru, Bolivia, Honduras, Australia Solanaceae (excluding those listed in (excluding Tasmania), Hawaiian appendices 3 and 42), Rosaceae (excluding Islands those listed in appendices 3 and 31) and Rutaceae (excluding those listed in appendices 4 to 8 ,39 ,45 and 56). -

Effect of Foliar Application of Boron with Calcium and Potassium on Quality and Yield of Mango Cv
Open Agriculture. 2019; 4: 98–106 Research Article Fatma Bibi*, Iftikhar Ahmad, Allah Bakhsh, Sidra Kiran, Subhan Danish, Hameed Ullah, Asif-ur-Rehman Effect of Foliar Application of Boron with Calcium and Potassium on Quality and Yield of Mango cv. Summer Bahisht (SB) Chaunsa https://doi.org/10.1515/opag-2019-0009 received September 8, 2018; accepted January 6, 2019 1 Introduction Abstract: Poor uptake of nutrients not only deteriorates Horticultural crops are seriously impacted by climatic the quality but also the yield of horticultural crops. Among variables (Normand et al. 2015). Due to changing climatic various macro and micronutrients, the role of K, Ca and conditions, tropic and sub-tropic areas of the world are B is very important. Therefore, balanced application and facing many challenges regarding mango productivity uptake of K and Ca and B can improve the quality and yield (Gerbaud 2012; Normand et al. 2015). Every stage of mango of mango trees. So, a field study was conducted with the phenological cycle (flowering, fruit growth, harvest/ hypothesis that combined application of K and Ca along vegetative growth and vegetative growth/rest) may get with B would be effective to improve yield and quality influenced by a change in temperature, precipitation, of Mango cv. Summer Bahisht (SB) Chaunsa. There were light, humidity and greenhouse gases (Christensen et al. two sources of Ca(CaCl2 and Ca(NO3)2) and three sources 2007). The anticipated climate changes and increasing of K(KNO3, K2SO4 and K-Citrate) combined with boric acid CO2 levels with global warming can result in greater (BA). -

Japan Market Desktop Report 8-11-19 Web.Pdf
Australian Institute for Business & Economics 24 June 2019 Evaluation of the potential to expand horticultural industries in Northern Australia CRCNA Project International Market Report – Japan Dr Shoufeng Cao A/Prof Damian Hine Professor Robert Henry Professor Neena Mitter CRCNA Project International Market Report – Japan Chapter Contents Background and purpose of the desktop research ...................................................................................... 1 1. Local production ........................................................................................................................................... 1 1.1 Mango production ......................................................................................................................................... 1 1.2 Avocado production ...................................................................................................................................... 1 1.3 Lychee production ........................................................................................................................................ 2 2. Import regulation .......................................................................................................................................... 2 2.1 Import protocol .............................................................................................................................................. 2 2.2 Ports of entry ............................................................................................................................................... -

NUTRITION and FERTILIZATION in MANGO. LITERATURE REVIEW Víctor Galán Saúco. Tropical Fruit Consultant Email: [email protected] Telephone: 34- 660331460
NUTRITION AND FERTILIZATION IN MANGO. LITERATURE REVIEW Víctor Galán Saúco. Tropical Fruit Consultant email: [email protected] Telephone: 34- 660331460 Table of contents Executive Summary 2 Introduction 3 Summary of interviews about fertilization in mango 3 Soil analysis 3 General recommendations for Fertilizing Mangos 5 Foliar analysis 9 General review 9 Sampling techniques 11 Interpreting leaf analysis 13 Nutrient extractions 15 Role of specific nutrients for mangos and ways of application 17 Introduction 17 Macronutrients 18 Micronutrients 24 Moment to apply fertilizers 27 Fertigation 29 Organic fertilization 31 Possibilities for future research projects in mango nutrition and fertilization 31 Summary of findings, general discussion and conclusion 32 Bibliography 34 Annex 1. List of interviewed people 44 Annex 2. Mango nutrition and fertilization survey 49 Annex 3. Soil analysis reported from the survey 52 Annex 4. Establishment of fertilization programs in different countries 60 Annex 5. Foliar Analysis. Survey results 61 Annex 6. Differences in leaf nutrient content depending in locations and phenological phases 65 Annex 7. Influence of nutrient relations in mango 67 Annex 8. Crop nutrient removal (kg/ha) per ton of production 69 Annex 9. Application of nutrients and tree phenology 71 Annex 10. Way of applying fertilizers 72 Annex 11. Research and/or interest in mango nutrition 74 1 Executive Summary The main objective of this report consists in providing assistance to mango growers in establishing an adequate fertilization program. To accomplish this objective a thorough literature review was complemented with a survey on mango nutrition and sent to mango producers and researchers all over the world, as well as information collected from different important fertilizers companies. -

Handbook for Agricultural and Fishery Products Import Regulations 2009
Handbook for Agricultural and Fishery Products Import Regulations 2009 February 2010 CONTENTS I. Products ....................................................................................................................1 1. Live Animals .............................................................................................................2 2. Meat and Prepared Products ...................................................................................7 3. Other Animal Products ...........................................................................................13 4. Fishery Products and Prepared Products .............................................................18 5. Dairy Products, etc. ...............................................................................................24 6. Plants, Resins and Vegetable Juices, etc. ............................................................28 7. Vegetables, Fruits and Prepared Products ............................................................33 8. Cereals and Prepared Products ............................................................................38 9. Sugars, Cocoa and Prepared Products .................................................................44 10. Spices ....................................................................................................................47 11. Oil Seeds and Prepared Products .........................................................................50 12. Various Prepared Foods ........................................................................................54 -

Can the Productivity of Mango Orchards Be Increased by Using
Scientia Horticulturae 218 (2017) 222–263 Contents lists available at ScienceDirect Scientia Horticulturae journal homepage: www.elsevier.com/locate/scihorti Review Can the productivity of mango orchards be increased by using high-density plantings? a,∗ b Christopher M. Menzel , M.D. Le Lagadec a Department of Agriculture and Fisheries, PO Box 5083, SCMC, Nambour, Qld. 4560, Australia b Department of Agriculture and Fisheries, 49 Ashfield Road, Bundaberg, Qld. 4670, Australia a r t i c l e i n f o a b s t r a c t Article history: Mango (Mangifera indica) trees are traditionally established at about 100–200 trees per ha and eventually Received 2 February 2016 grow into large specimens 10 m tall or more, making spraying and harvesting difficult. It also takes a long Received in revised form time to recover the initial costs of establishing and maintaining the orchard. There has been considerable 16 November 2016 interest in planting orchards up to 4000 trees per ha to take advantage of early production and to increase Accepted 28 November 2016 economic returns. However, trees planted at high density soon begin to crowd and shade each other and production falls. We reviewed the performance of high-density orchards in different growing areas, Keywords: and the role of dwarfing cultivars and rootstocks, tree canopy management and the growth regulator, Mango paclobutrazol to control tree growth. There has been no general agreement on the optimum planting Tree growth Yield density for commercial orchards which vary from 200–4000 trees per ha in different experiments. Some High-density plantings potential dwarfing material has been developed in India and elsewhere, but these cultivars and rootstocks Pruning have not been widely integrated into high-density orchards. -

Appendix 3. Mango Objective Reporting
Final Report Data collection to facilitate supply chain transparency - stage 2 Trevor Dunmall Australian Mango Industry Association Ltd Project Number: MG12007 MG12007 This project has been funded by Horticulture Innovation Australia Limited using the mango industry levy and funds from the Australian Government. Horticulture Innovation Australia Limited (HIA Ltd) makes no representations and expressly disclaims all warranties (to the extent permitted by law) about the accuracy, completeness, or currency of information in Data collection to facilitate supply chain transparency - stage 2. Reliance on any information provided by HIA Ltd is entirely at your own risk. HIA Ltd is not responsible for, and will not be liable for, any loss, damage, claim, expense, cost (including legal costs) or other liability arising in any way (including from HIA Ltd or any other person’s negligence or otherwise) from your use or non-use of Data collection to facilitate supply chain transparency - stage 2, or from reliance on information contained in the material or that HIA Ltd provides to you by any other means. ISBN 0 7341 3551 3 Published and distributed by: Horticulture Innovation Australia Limited Level 8, 1 Chifley Square Sydney NSW 2000 Tel: (02) 8295 2300 Fax: (02) 8295 2399 © Copyright 2015 Contents Summary ................................................................................................................................... 3 Keywords .................................................................................................................................. -

List of Ban-Lifted Plant Items and Key Conditions
LIST OF BAN-LIFTED PLANT ITEMS AND KEY CONDITIONS (MEET THE STANDARDS SET BY THE MINISTER OF AGRICULTURE, FORESTRY AND FISHERIES) Edited by Japan Fresh Produce Import and Safety Association (P.I.S.A). March, 2015 FRESH FRUITS COUNTRIES / DISTRICTS BAN-LIFTED YEAR TARGET PESTS TREATMENT OR OTHER MEASURES MEANS OF CONVEYANCE ITEMS CULTIVARS UNITED STATES HAWAIIAN ISLANDS PAPAYA SOLO April, 1969 ①MEDITERRANEAN FRUIT FLY Vapor Heat Treatment: SHIP CARGO OF AMERICA MANGO KEITT May, 2000 ②ORIENTAL FRUIT FLY COMPLEX The fruits shall be disinfested by using saturated vapor at vapor heat treatment facilities till the temperature of the innermost fruit pulp reaches 47.2℃. AIR CARGO HADEN ③MELON FLY AIR HAND BAGGAGE EXCLUDING CHERRY 1.BING ①January, 1978 CODLING MOTH Methyl Bromide Fumigation SHIP CARGO HAWAIIAN IS. 2.LAMBERT ②December, 1986 ① Of cultivars 1~10 AIR CARGO 3.VAN Dosage Temperature Duration of exposure Ratio of fruit capacity 4.RAINIER May, 1992 32g/㎥ 22℃ or above 2 hours 50% or less 5.GARNET January, 1995 40g/㎥ 17~22℃ or below 2 hours 50% or less 6.TULARE October, 1996 48g/㎥ 12~17℃ or below 2 hours 50% or less 7.BROOKS 64g/㎥ 6~12℃ or below 2 hours 50% or less 8.LAPIN July, 1999 ② Or all the cultivars including cultivars 1~10, and 11 9.SWEET HEART The production of the concentration of methyl bromide at the Dosage Temperature Duration of exposure 10.CHELAN May, 2001 fumigation facilities by duration exposure 11.EXCEPT ABOVES October, 2001 64g/㎥ 6~12℃ or below 2 hours or more 61.9 or up ALL VARIETY Jun,2009 1. -

Carmakal Full Inventory
Tree Name/Variety Additional Info Abui Abui/Pouteria Ciamito Acai Akee Alexander Allspice Aloe Aloe/Aloe barbadensis Medicinal Aloe Apple Araza Araza/Eugenia Stipitata Areca Atemoya Atemoya Priestley Atemoya/Annona Hybrids/Gefner Atemoya/Annona Hybrids/Lisa Atemoya/Annona Hybrids/Priestly Atemoya/Bradley Atemoya/Gefner Atemoya/Lisa Atemoya/Nuathong Atemoya/Phet Pakchong Avocado Avocado/Bacon Avocado/Bernecker Avocado/Brogdon Cold Hardy Avocado/Brogdon Avocado/Catalina Not Cold Hardy Avocado/Catalina Avocado/Choquette Med Avocado/Choquette Avocado/Custers Red Avocado/Day Cold Hardy Avocado/Day Avocado/Donnie Not Cold Hardy Avocado/Fairchild Red Avocado/Florida Haas Cold Hardy Avocado/Florida Hass Avocado/Fuerte Avocado/Hall Med Avocado/Hall Avocado/Heath Avocado/Lula Med Avocado/Lula Avocado/Marcus Pumpkin Med Avocado/Marcus Pumpkin Avocado/Maria Not Cold Hardy Avocado/Mexicola Avocado/Miguel Avocado/Monroe Med Avocado/Monroe Avocado/Oh La La Cold Hardy Avocado/Oro Negro Not Cold Hardy Avocado/Oro Negro Avocado/Pollack Not Cold Hardy Avocado/Pollock Avocado/Russel Avocado/Russell Not Cold Hardy Avocado/Simmonds Not Cold Hardy Avocado/Simmonds Avocado/Spice Park Red Avocado/Thomson Red Not Cold Hardy Avocado/Utuado Not Cold Hardy Avocado/Wilson Seedless Avocado/Winter Mexican Cold Hardy Avocado/Winter Mexican Avocado/Wurtz Banan Banan/Musa Sp. Ice Cream Banan/Musa Sp. Dwarf Cavandish Banan/Musa Sp. Dwarf Nam Wah Banan/Musa Sp. Plantain Banana Banana/Basjo Banana/Double Banana Banana/Dwarf Cavendish Banana/Dwarf Nino Banana/Dwarf Red Banana/Ele -

(Annex3) (Last Updated: 23 December 2019) Note: Underlined Region/Countries Or Plants Will Be Added. Strikethrough Countries
(Annex3) (Last updated: 23 December 2019) Proposed revision of List of the Import Prohibited Plants (Annexed Table 2 of the Ordinance for Enforcement of the Plant Protection Act) Note: Underlined region/countries or plants will be added. Strikethrough countries will be deleted. Item Region/countries Plants Quarantine pests No 1 [Middle East] Yemen, Israel, Iran, Saudi Arabia, Syria, Fresh fruits of the following plants: Ceratitis capitata (Mediterranean fruit fly) Turkey, Jordan, Lebanon, akee (Blighia sapida), avocado (Persea americana) (excluding those listed in [Europe] Albania, Italy, Austria, Netherlands, Republic of Appendix 60 and 64), Malay gooseberry (star berry) (Phyllanthus acidus), allspice North Macedonia, Cyprus, Greece, Croatia, Kosovo, (Pimenta dioica (syn. Pimenta officinalis)), olive (Olea europaea), cashew Switzerland, Spain, Slovenia, Serbia, Germany, Hungary, (Anacardium occidentale), kiwi fruit (Actinidia deliciosa, A. chinensis)), yellow France, Belgium, Bosnia and Herzegovina, Portugal, oleander(Thevetia peruviana (syn. T. nereifolia)), Cucumis dipsaceus, Coccinia Former Yugoslav Republic of Macedonia, Malta, microphylla, Corallocarpus ellipticus, carambola (Averrhoa carambola), Montenegro, Russia, pomegranate (Punica granatum), jaboticaba (Plinia cauliflora (syn. Eugenia [Africa] Africa, cauliflora, Myrcia jaboticaba)), broad bean (Vicia faba), alexandrian laurel [Latin America] Bermuda islands, Argentina, Uruguay, (Calophyllum inophyllum), date palm (Phoenix dactylifera), nance (Byrsonima Ecuador, El Salvador, -
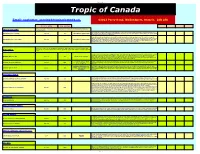
View It Here!!
Full List 2021 Tropic of Canada Email- [email protected]. 63023 Perry Road, Wellandport, Ontario. L0R 2J0 FULL SIZE - METERS IDEAL MIN TEMP Indoor or outside . If container grown bring inside for fall harvest. Hardy Kiwi will ripen on CATEGORY KIWI your windowsill. Issai is less vigorous than other kiwi's which enables it to be grown in gardens with limited space. This has been known to ACTINIDIA arguta 'ISSAI' 3m x 2m -10'C SELF FERTILE, HARDY KIWI fruit in its first year. Blossom in Spring-fall fruiting. Bite sized 'fuzz' free. As a patio plant, requires support, set outside during the summer and move indoors in the winter. Fruits will reach full-size by mid-season, but will not fully ripen until late in the growing season. They will keep longer if picked just slightly before fully ripe and refrigerated. A variety that is early ripening and produces abundant crops of medium ACTINIDIA arguta 'PROLIFIC' 4m x 3m -10'C SELF FERTILE, HARDY KIWI to large, sweet and tasty fruit. The cultivar is sometimes used as a pollinator for other female cultivars. Fruits are flavourful with reddish/violet on the skin and flesh when ripe. Minimum chilling requirements; 300 hours. These Kiwi have smooth- skinned fruits so there is no need to peel the skin of the fruits unlike the fuzzy fruit species Ananas are easy to grow, drought tolerant plants. Although they are not very cold tolerant PINEAPPLE they can be overwintered indoors as a house plant. The plants will fruit in a container. All self- fertile. -

Supply Chain Collaboration in the Management of Nam Dok Mai Mango Exports from Thailand to Japan
SUPPLY CHAIN COLLABORATION IN THE MANAGEMENT OF NAM DOK MAI MANGO EXPORTS FROM THAILAND TO JAPAN by SUPAJIT PANICHSAKPATANA A thesis submitted to the University of Newcastle upon Tyne in partial fulfillment for the degree of Doctor of Philosophy NEWCASTLE UNIVERSITY BUSINESS SCHOOL University of Newcastle upon Tyne May 2013 ABSTRACT This thesis aims to develop the supply chain collaboration in the management of fresh Nam Dok Mai mangoes for exports to Japan. To accomplish the research aim, three main objectives are addressed; 1) to provide an overview of existing supply chain of Nam Dok Mai mangoes in Thailand and to identify strengths and weakness in the supply chain; 2) to analyse supply chain collaboration between growers and exporters in the production of mangoes for export to Japan; and 3) to provide recommendations to the government and related agencies on sufficient supply chain management for fresh mango exports. Following a theoretical review, the study employs a conceptual framework for the study of collaborative supply chain that differs from the traditional concepts used in the manufacturing industries literature. The thesis framework presents concepts of supply chain collaboration used for an agro-food industry focusing on the perishable products. The discussion and analysis based on the six case studies of mango export companies which are the main exporters in Thailand. Semi-structured interviews are conducted to gather data from growers and exporters. A cross-case analysis is applied to examine the collaboration and to compare similarities and differences among six companies. The developed theoretical concepts of supply chain collaboration are discussed in the cross-case analysis to determine the good collaborative practices between growers and exporters in each case study.