Development of Electronic Testing Devices That Detect Peripheral Neuropathies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Neurophysiology Board Review Q&A
Clinical Neurophysiology Board Review Board Clinical Neurophysiology Clinical Neurophysiology Board Review Q&A Clinical Puneet K. Gupta, MD, MSE • Pradeep N. Modur, MD, MS • Srikanth Muppidi, MD his high-yield, illustrated clinical neurophysiology board review is a comprehen- Neurophysiology sive resource for assessing and refining the knowledge tested on multiple board Texaminations. Written by authors who are collectively board certified in all of the areas covered, the book is a valuable study tool for candidates preparing for certifica- tion or recertification in clinical neurophysiology, neuromuscular medicine, epilepsy, Board Review sleep medicine, and neurology. Using structured question formats typically encountered on boards, this comprehensive review allows users to assess their knowledge in a wide range of topics, provides rationales for correct answers, and explains why the other choices are incorrect. A unique “Pearls” section at the end of the book allows for quick review of the most important concepts prior to exam day. Clinical Neurophysiology Board Review Q&A contains 801 questions with answers and detailed explanations. The book is divided into eight chapters covering anatomy Q and physiology, electronics and instrumentation, nerve conduction studies and EMG, & EEG, evoked potentials and intraoperative monitoring, sleep studies, ethics and safety, and advanced topics including QEEG, MEG, TES, autonomic testing, and more. A Liberal use of image-based questions illustrating the full spectrum of neurophysiologic & tests and findings build interpretive skills. Questions are randomized and include Q A both case-related questions in series and stand-alone items to familiarize candidates Gu with the question types and formats they will find on the exam. -

The Peripheral Nerves: Update on Ultrasound and Magnetic Resonance Imaging I
The peripheral nerves: update on ultrasound and magnetic resonance imaging I. Möller1, M. Miguel2, D.A. Bong1, F. Zaottini3, C. Martinoli4 1Instituto Poal de Reumatologia, ABSTRACT of the nerve along its trajectory along University of Barcelona, Spain, The motor and sensory branches of with immediate one-to-one compari- and EULAR Working Group Anatomy the somatic peripheral nervous system son with the contralateral structures (6, for the Image; (PNS) can be visualised by different im- 7). In addition, US-guidance has led to 2Department of Pathology and Experimental Therapeutics, Human aging systems. This article focuses on the development of a variety of inter- Anatomy and Embryology Unit, imaging of peripheral nerves by mag- ventional procedures. The use of US is University of Barcelona, Spain; netic resonance imaging (MRI) and becoming widespread in providing ac- 3Department of Health Sciences, high-resolution ultrasound (US). The curate and safe regional anesthesia as DISSAL, University of Genoa; anatomic basis of the peripheral nerve well as focal and regional pain manage- 4 Department of Health Science, image, common pathologies and clini- ment. It has also becoming an increas- University of Genoa, Ospedale cal value of US and MRI imaging of pe- ingly important component of muscu- Policlinico San Martino, Genoa, Italy. ripheral nerves are reviewed. loskeletal specialties such as physical Ingrid Möller, MD medicine and rehabilitation and sports Maribel Miguel, MD David A. Bong, MD Introduction medicine. Federico Zaottini, MD Nerve pathology may be a cause of Carlo Martinoli, MD chronic pain and disability. The initial Anatomical considerations Please address correspondence to: diagnostic evaluation of the periph- The PNS includes spinal nerves that Dr David A. -

EMG (Electromyography) And/Or NCS (Nerve Conduction Studies)
Tempe ñ Phoenix ñ Gilbert ñ Scottsdale ñ Peoria ñ Show Low PHONE: (480) 962-0071 www.SonoranSpine.com ñ www.SpineResearch.org Patient Name: Date: Date of Birth: S O N O R A N S P I N E -- R E F E R E N C E Electromyography and Nerve Conduction Studies An electromyogram (EMG) measures the electrical The electrode will be moved a number of times to activity of muscles at rest and during contraction. record the activity in different areas of the muscle or in Nerve conduction studies (NCS) measure how well and different muscles. how fast the nerves can send electrical signals. Nerve conduction studies If you have leg pain or numbness, you may undergo Several electrodes are attached to your skin. Several these tests to determine how much your nerves are quick electrical pulses are given to the nerve, and the being affected. These tests check how well the spinal time it takes for the muscle to contract in response to nerves and the nerves in your arms and legs are the electrical pulse is recorded. The speed of the working and whether there is nerve irritation or response is called the conduction velocity. The same damage. They do not test for pain. nerves on the other side of the body may be studied for comparison. When the test is done, the electrodes Why It Is Done are removed. An EMG is done to determine muscle tissue or nerve damage. It can find the cause of weakness, paralysis, How It Feels or muscle twitching. -

Nerve Conduction Studies: Essentials and Pitfalls in Practice
NERVE CONDUCTION STUDIES: J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2005.069138 on 16 June 2005. Downloaded from ESSENTIALS AND PITFALLS IN PRACTICE A Mallik, A I Weir ii23 J Neurol Neurosurg Psychiatry 2005;76(Suppl II):ii23–ii31. doi: 10.1136/jnnp.2005.069138 neurologist has sent a patient for nerve conduction studies (NCS) and has received the report, but what does it mean? We hope to remove some of the mysteries that may Asurround NCS. The techniques and how they are affected by disease are described in general terms. These principles can be applied to specific conditions discussed elsewhere. We also discuss the numerous pitfalls that may be encountered with NCS. Understanding these basic concepts will allow you to get the most from your clinical neurophysiology department. NCS are only part of a complete peripheral neurophysiological examination (PNE) and are frequently accompanied by a needle electromyogram (EMG). The combination of both techniques and those detailed in other articles in this issue are often required for a complete diagnostic study. The process of choosing the appropriate tests is the responsibility of the clinical neurophysiologist (CN) and not the referring doctor and is planned as a dynamic series of steps designed to answer specific questions about nervous system function raised by the clinical picture. c ABBREVIATIONS Clinical neurophysiologists can employ a confusing number of terms and abbreviations. Box 1 lists the ones we use frequently. WHAT DO YOU TELL THE PATIENT ABOUT THE TESTS? NCS involve activating nerves electrically with small safe pulses over several points on the skin of copyright. -

Patient Education Electromyogram (EMG) Nerve Conduction Study
Patient Education Electromyogram (EMG) Nerve Conduction Study Explanation of Test Your doctor has ordered a test called an EMG. EMG stands for electromyogram which loosely translated means electrical testing of muscles, but it has come to mean electrical testing of nerves and muscles. A specialist, the electromyographer, who has specialized training in the field of electromyography, performs the EMG. EMG is an in-office procedure that does not require hospitalization. On average, an EMG takes anywhere between 30 minutes and 2 hours, depending on how extensive your test is ordered to be. It can be done at any time during the day and, with few exceptions, does not require any special preparation. Sometimes EMGs are thought to be a treatment of some sort, or a type of acupuncture. This is not true. An EMG is only a test, much like an EKG or an x-ray. EMGs are usually ordered when you are having problems with your muscles or nerves. EMG’s test the muscles and nerves of your arms and legs to identify problems. Weakness of your muscles or “fatigue” (tiredness) may indicate nerve or muscle disease and require an EMG. Usually combined with nerve conduction studies (NCS), EMG’s are the most important diagnostic tests for the evaluation of neuropathy and myopathy (nerve and muscle disease). These tests are performed on motor and sensory nerves, but only large myelinated fibers can be evaluated by nerve conduction studies. EMG’s help diagnose conditions such as in carpal tunnel syndrome, polyneuropathy (as seen with diabetes), nutritional deficiencies, autoimmune processes, or degradation of the myelin (a type of coating around the nerve axon which allows the electrical signal to travel faster), as seen in Guillan Barre´ Syndrome. -

M.Sc. Medical Physiology Will Be of Six Semesters Spread Over Three Years
KRISHNA INSTITUTE OF MEDICAL SCIENCES, “DEEMED TO BE UNIVERSITY”, KARAD. KRISHNA INSTITUTE OF MEDICAL SCIENCES PROGRAMME NAME: M. Sc. Medical Physiology. CHOICE BASED CREDIT SYSTEM (CBCS). PROGRAMME CODE: 1503 COURSE NAME: Paper I, II, III, and IV. COURSE CODE: 1503-11, 12, 13, 14. PREAMBLE: The aim of the course is to prepare PG students who shall 1) Teach and train future under-graduate & post-graduate medical students Human Physiology in Medical Colleges and Research Institutions. 2) Carry out & guide research & in academics, can go for higher qualifications like Ph.D. in Physiology & contribute to advancement of the subject. 3) Earn to placements in Medical teaching institute, research laboratories run by the government and the corporate sector & organize & manage administrative responsibilities for routine day to day departmental work. Objectives: At the end of course student should be able to achieve: A-Domain: 1) Cognitive domain: All the systems of the body should be studied with respect to: a) Historical aspect b) Evolution and development c) Comparative physiology d) Structure-gross and electron microscopic and functions at cellular level e) Qualitative and quantitative aspects. f) Regulating mechanisms g) Variations in physiological and pathological conditions h) Applied physiology i) Recent advances 2) Psychomotor domain: P.G. students should be able- a) To perform human and animal (mammalian, amphibian) experiments: Hematology, Experiments based on biophysical principles. b) To acquire history taking and clinical examination skills. 3) Affective domain a) The P.G. students should develop communication skills to interact with students, colleagues, superiors and other staff members. b) They should be able to work as a member of a team to carry out teaching as well as research activities c) They should have right attitude toward teaching profession B) Global Competencies & Employability: The student should get employment in the following branches related to the course and should acquire the competency for the same. -

EMG/Nerve Conduction Studies Electrodiagnostics
Will I experience pain? EMG/Nerve Conduction Studies This is the most common question. The nerve conduction study mimics the sensation of receiving a shock from Electrodiagnostics static electricity. The EMG resembles a sharp poke, followed by a deep muscle ache. The test can be About our physician uncomfortable at times but not unbearable. Some mild muscle soreness may persist for a short period of time. Matthew J. Smith, MD Dr. Smith received his EMG/NCS training during his Physical Medicine & Rehabilitation residency at What are the risks of this test? Stanford University and during his fellowship at the University of Michigan Electrodiagnostics Laboratory. He As with all medical procedures, there are risks. Anytime completed the American Association of Neuromuscular the skin is broken by a needle there is a risk of infection, & Electrodiagnostic Medicine’s accreditation process in pain at the site, swelling, bruising or bleeding. May 2001. The most serious risk of procedures in the upper back and chest is a pneumothorax (collapsed lung), which could lead to hospitalization and need for further treatment. Though rare, complications can occur and your doctor will address these prior to your procedure. Diagnosing the symptoms of: Numbness or tingling Pain Muscle weakness Low back pain Sciatica Carpal tunnel syndrome Radiculopathy www.universityorthopedics.com 401-402-1065 1598 South County Trail East Greenwich, RI 02818 What is an EMG/NCS? How long will the test take? How are the tests performed? EMG or electromyography is a study that examines the The test will take 30-45 minutes typically. Occasionally, the Needle EMG nerves and muscles of the body. -

Practice Parameter for Repetitive Nerve Stimulation and Single Fiber
PRACTICE PARAMETER FOR REPETITIVE NERVE STIMULATION AND SINGLE FIBER EMG EVALUATION OF ADULTS WITH SUSPECTED MYASTHENIA GRAVIS OR LAMBERT-EATON MYASTHENIC SYNDROME: SUMMARY STATEMENT American Association of Electrodiagnostic Medicine Developed by the AAEM Quality Assurance Committee (1997-2000): primary authors: Faye Y. Chiou- Tan, MD; Richard W. Tim, MD; and James M. Gilchrist, MD; chairs: Cheryl F. Weber, MD (1998), John R. Wilson, MD (1999-2000); committee members: Timothy J. Benstead, MD, FRCP (C), Arlene M. Braker, MD, James B. Caress, MD, Sudhansu Chokroverty, MD, FRCP, Earl R. Hackett, MD, Robert L. Harmon, MD, MS, Bernadette A. Hughes, MD, Milind J. Kothari, DO, Tim Lachman, MD, Richard I. Malamut, MD, Cristina M. Marciniak, MD, Robert G. Miller, MD, Kevin R. Nelson, MD, Richard K. Olney, MD, Atul T. Patel, MD, Caroline A. Quartly, MD, FRCP(C), and Karen S. Ryan, MD. Key words: Lambert-Eaton myasthenic syndrome; single fiber electromyography; myasthenia gravis; neuromuscular junction; repetitive nerve stimulation Correspondence to: American Association of Electrodiagnostic Medicine, 421 First Avenue SW, Suite 300 East, Rochester, MN 55902; e-mail: [email protected] 2 INTRODUCTION Myasthenia gravis is an autoimmune disorder that affects approximately 3 out of 10,000 people. Myasthenia gravis affects the neuromuscular junction (NMJ) and produces weakness of voluntary muscles. In some cases, it may be associated with tumors of the thymus (a tissue of the immune system). It also may be associated with thyrotoxicosis, rheumatoid arthritis, systemic lupus erythematosus, and other immune system disorders. In many cases, no other disorder is identified. Myasthenia gravis (MG) is most common in adult women. -

Neuroanatomy for Nerve Conduction Studies
Neuroanatomy for Nerve Conduction Studies Kimberley Butler, R.NCS.T, CNIM, R. EP T. Jerry Morris, BS, MS, R.NCS.T. Kevin R. Scott, MD, MA Zach Simmons, MD AANEM 57th Annual Meeting Québec City, Québec, Canada Copyright © October 2010 American Association of Neuromuscular & Electrodiagnostic Medicine 2621 Superior Drive NW Rochester, MN 55901 Printed by Johnson Printing Company, Inc. AANEM Course Neuroanatomy for Nerve Conduction Studies iii Neuroanatomy for Nerve Conduction Studies Contents CME Information iv Faculty v The Spinal Accessory Nerve and the Less Commonly Studied Nerves of the Limbs 1 Zachary Simmons, MD Ulnar and Radial Nerves 13 Kevin R. Scott, MD The Tibial and the Common Peroneal Nerves 21 Kimberley B. Butler, R.NCS.T., R. EP T., CNIM Median Nerves and Nerves of the Face 27 Jerry Morris, MS, R.NCS.T. iv Course Description This course is designed to provide an introduction to anatomy of the major nerves used for nerve conduction studies, with emphasis on the surface land- marks used for the performance of such studies. Location and pathophysiology of common lesions of these nerves are reviewed, and electrodiagnostic methods for localization are discussed. This course is designed to be useful for technologists, but also useful and informative for physicians who perform their own nerve conduction studies, or who supervise technologists in the performance of such studies and who perform needle EMG examinations.. Intended Audience This course is intended for Neurologists, Physiatrists, and others who practice neuromuscular, musculoskeletal, and electrodiagnostic medicine with the intent to improve the quality of medical care to patients with muscle and nerve disorders. -

Peripheral Nerve Function Changes Due to Hypoxia in Obstructive Sleep Apnea
Original Article / Özgün Makale DO I: 10.4274/jtsm.galenos.2019.27147 Journal of Turkish Sleep Medicine 2019;1:37-42 Peripheral Nerve Function Changes Due to Hypoxia in Obstructive Sleep Apnea Obstrüktif Uyku Apne’de Hipoksiye Bağlı Periferal Sinir Fonksiyonu Değişiklikleri Aynur Yılmaz Avcı, Suat Avcı* Başkent University Faculty of Medicine, Department of Neurology, Ankara, Turkey *Başkent University Faculty of Medicine, Department of Otolaryngology, Ankara, Turkey Abstract Öz Introduction: Chronic hypoxia is known to be one of the risk factors Amaç: Periferik nöropati için risk faktörlerinden birinin kronik hipoksi for peripheral neuropathy. However, the effect of intermittent hypoxia olduğu bilinmektedir. Bununla birlikte, aralıklı hipoksinin periferal on peripheral nerves is not fully understood. This study evaluated the sinirler üzerindeki etkisi tam olarak anlaşılamamıştır. Bu çalışmada relation between intermittent hypoxia and peripheral nerve function in Obstrüktif Uyku Apnesi (OSA) hastalarında aralıklı hipoksi ve periferik Obstructive Sleep Apnea (OSA) patients. sinir fonksiyonu arasındaki ilişki değerlendirildi. Materials and Methods: In this retrospective study, 86 patients who Gereç ve Yöntem: Bu retrospektif çalışmaya polisomnografi (PSG) ve underwent polysomnography (PSG) and electroneuromyography were elektronöromiyografi uygulanmış 86 hasta alındı. Periferik sinirleri ve enrolled. Participants with diseases affecting peripheral nerves and akciğer fonksiyonlarını etkileyen hastalıkları olan katılımcılar çalışma lung function -

What Is an EMG/Nerve Conduction Study?
Information for patients What is an EMG/Nerve Conduction Study? Department of Neurophysiology We must obtain your consent for any procedure beforehand. Staff will explain all the risks, benefits and alternatives before they ask for your consent. If you are unsure about any aspect of the proposed procedure, please do not hesitate to ask for moreinformation. What is an EMG/Nerve Conduction Study? This is a routine test performed in specialist hospitals. The EMG (electromyography) records the electrical impulses that your muscles produce. The Nerve Conduction test measures the speed at which impulses travel along a nerve. These tests help us to work out how well your nerves and muscles are functioning. They are often referred to collectively as an EMG test and may incorporate one or both of the above techniques. What does EMG stand for? Electro- The electrical impulses that are being measured. Myo- Muscle. Graphy- The way the results of the test are presented. How do EMG/Nerve Conduction studies work? The Nerve Conduction Study records how fast nerves send messages to the brain and back. In the test, we stimulate the nerves using electrodes placed on the surface of a person’s skin. We can then record how fast the impulse travels to another point, where it is recorded using a surface electrode placed on the skin (on rare occasions a fine needle electrode in the muscle may have to be used to take a recording). This tells us how fast the nerve is working. 2 EMG studies the electrical activity of the muscles. This is usually recorded using a small needle electrode inserted through the skin into the muscle, which produces a short pinprick sensation. -
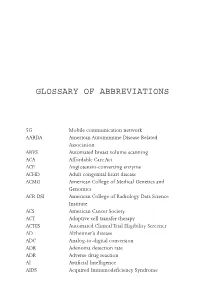
Glossary of Abbreviations
GLOSSARY OF ABBREVIATIONS 5G Mobile communication network AARDA American Autoimmune Disease Related Association ABVS Automated breast volume scanning ACA Affordable Care Act ACE Angiotensin-converting enzyme ACHD Adult congenital heart disease ACMG American College of Medical Genetics and Genomics ACR DSI American College of Radiology Data Science Institute ACS American Cancer Society ACT Adoptive cell transfer therapy ACTES Automated Clinical Trial Eligibility Screener AD Alzheimer’s disease ADC Analog-to-digital conversion ADR Adenoma detection rate ADR Adverse drug reaction AI Artificial Intelligence AIDS Acquired Immunodeficiency Syndrome II GLOSSARY OF ABBREVIATIONS ALS Amyotrophic Lateral Sclerosis ALU Arithmetic logic unit AMD Age-related macular degeneration AMIGO Advanced Multimodality Image Guided Operating Suite AMP Association for Molecular Pathology ANA Antinuclear antibodies ANCA Antineutrophil cytoplasmic antibodies ANN Artificial Neural Network or Memory Network APC Antigen-presenting complex APHA American Public Health Association API Application Programming Interface App Application software APU Accelerated processing unit ARB Angiotensin II receptor blockers As Adenine ASCA Administrative Simplification Compliance Act ASCO Adaptive Stem Cell Optimization ASIC Application Specific Integrated Circuit ASR Automated Speech Recognition AUC Area under the receiver operating characteristic curve AUROC see AUC AUTOMAP Automated transform by manifold approximation (MRI) B1 B lymphocyte BCC Basal cell carcinoma (skin cancer)