2015 Scheidecker Bardet Biedt
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rod-Cone Dystrophy Associated with the Gly167asp Variant in PRPH2
Rod-cone dystrophy associated with the Gly167Asp variant in PRPH2 Rola Ba-Abbad, FRCS, PhD1,2, Anthony G. Robson, PhD1,2, Becky MacPhee, BSc2, Andrew R. Webster, MD(Res), FRCOpth1,2, Michel Michaelides, MD(Res), FRCOphth 1,2 1. UCL Institute of Ophthalmology, University College London, London, UK 2. Moorfields Eye Hospital, London, UK Declaration of interest statement: the authors report no conflict of interest. Corresponding Author: Professor Michel Michaelides, MD(Res), FRCOphth UCL Institute of Ophthalmology, London, EC1V 9EL, United Kingdom Email: [email protected] Phone number: +44 (0) 20 7608 6800 Peripherin 2-associated retinopathies are phenotypically heterogenous and can present as autosomal dominant retinitis pigmentosa, cone-rod dystrophy, various forms of macular and pattern dystrophy, or recessive retinopathy1,2. We report a case of rod-cone dystrophy associated with the variant c.500G>A, p.(Gly167Asp) in PRPH2 (OMIM 179605), which was previously reported to cause autosomal dominant butterfly-shaped pigment dystrophy of the fovea in a three-generation pedigree (MIM 169150)3. A 66-year old British woman of European ancestry was referred to the inherited retinal disorders clinic with bilateral pigmentary retinopathy, and a 5-year history of nyctalopia. There were no knowingly affected family members; her late father and mother had normal vision in their sixties and eighties respectively, and the patient’s two children had no symptoms in their third decade of life. Previously, she underwent laser refractive surgery for myopia, bilateral cataract extraction and laser posterior capsulotomy. On examination, the Snellen visual acuity was 20/30 in the right eye, and 20/80 in the left eye; and color vision (Ishihara plates) was normal bilaterally. -

DJO Macular Dystrophy in a Post LASIK Patient
DJO Vol. 30, No. 3, January-March 2020 Case Report Macular Dystrophy in a Post LASIK Patient Sanjana Vatsa, Shana Sood Dr. Agarwal Eye Hospital, Chennai, Tamil Nadu, India LASIK (Laser Assisted Insitu keratomileusis) is the most commonly performed refractive surgery worldwide. A detailed pre operative and post operative evaluation of the anterior and posterior segment is a must. A 35 year old male patient with a history of LASIK surgery done 13 years back presented to us with complaint of painless, progressive diminution of vision in both eyes from past Abstract 2 years. Dilated retinal examination showed bulls eye maculopathy in both eyes. Macular OCT showed gross reduction in central foveal thickness. ERG showed marked reduction in photopic responses suggestive of a cone dystrophy. Treatment aims at alleviating the symptoms and use of low vision aids. Genetic counselling may be of benefit for affected individuals and their families. Delhi J Ophthalmol 2020;30;60-62; Doi http://dx.doi.org/10.7869/djo.529 Keywords: LASIK, Bulls eye maculopathy, cone dystrophy, genetic counselling. Introduction LASIK is the most popular and commonly performed refractive surgery worldwide.1 Along with anterior segment, a detailed evaluation of the posterior segment is a must on follow up visits to rule out any retinal lesions such as degenerations, dystrophies, maculopathy etc; as these can occur irrespective of any procedure performed. Case Report A 35 year old male patient came to us with a history of LASIK surgery done 13 years back in both eyes for a power of -7.0D sphere. Patient was comfortable with his vision after surgery and had no complaints for 11 years, after which he noticed blurring of vision in both eyes (more in the left eye). -

The Role of Erg/Vep and Eye Movement Recordings in Children with Ocular Motor Apraxia
THE ROLE OF ERG/VEP AND EYE MOVEMENT RECORDINGS IN CHILDREN WITH OCULAR MOTOR APRAXIA FATIMA S. SHAWKAT, CHRISTOPHER M. HARRIS, DAVID S. I. TAYLOR and ANTHONY KRISS London SUMMARY several reports of OMA or saccade failure occurring Ocular motor apraxia (OMA) is characterised by an congenitally, with no other clinical entity?-5 How intermittent inability to initiate voluntary sacca des, and ever, it can also occur as part of a wider neurological a failure to produce optokinetic and vestibular quick disorder: for example with structural brain abnorm phases. Some patients have no other abnormalities alities, such as agenesis of the corpus callosum6 and (idiopathic OMA), whereas in others it appears vermis hypoplasia;7 with neurodegenerative condi associated with a variety of neurological conditions tions;8 and with acquired neurological disease such as which may affect the sensory visual pathway. Electro posterior fossa tumours,9 ataxia telangiectasia,lO retinograms (ERGs), flash and pattern visual evoked fronto-parietal lesions,l1.l2 occipital cortex lesions,13 potentials (VEPs) and eye movements were assessed in cerebellar and brains tern neoplasm14 and olivoponto 53 children with OMA (age range 17 days to 14 years) cerebellar degeneration. 15.16 The inability to gener to determine their efficacy in helping to distinguish ate saccades often leads to the development of between idiopathic and non-idiopathic cases. Seven patients (13.2%) had idiopathic OMA and the remain compensatory behaviour to shift direction of gaze; ing 46 (86.8%) had other associated clinical conditions. this includes headthrusting, blinking and tilted head All patients had episodes of absent quick phases ('lock posture, which enables the use of vertical eye up') during optokinetic nystagmus (OKN) and/or movements that are usually unaffected. -

Progressive Cone and Cone-Rod Dystrophies
Br J Ophthalmol: first published as 10.1136/bjophthalmol-2018-313278 on 24 January 2019. Downloaded from Review Progressive cone and cone-rod dystrophies: clinical features, molecular genetics and prospects for therapy Jasdeep S Gill,1 Michalis Georgiou,1,2 Angelos Kalitzeos,1,2 Anthony T Moore,1,3 Michel Michaelides1,2 ► Additional material is ABSTRact proteins involved in photoreceptor structure, or the published online only. To view Progressive cone and cone-rod dystrophies are a clinically phototransduction cascade. please visit the journal online (http:// dx. doi. org/ 10. 1136/ and genetically heterogeneous group of inherited bjophthalmol- 2018- 313278). retinal diseases characterised by cone photoreceptor PHOTORECEPTION AND THE degeneration, which may be followed by subsequent 1 PHOTOTRANSDUCTION CASCADE UCL Institute of rod photoreceptor loss. These disorders typically present Rod photoreceptors contain rhodopsin phot- Ophthalmology, University with progressive loss of central vision, colour vision College London, London, UK opigment, whereas cone photoreceptors contain 2Moorfields Eye Hospital NHS disturbance and photophobia. Considerable progress one of three types of opsin: S-cone, M-cone or Foundation Trust, London, UK has been made in elucidating the molecular genetics L-cone opsin. Disease-causing sequence variants 3 Ophthalmology Department, and genotype–phenotype correlations associated with in the genes encoding the latter two cone opsins University of California San these dystrophies, with mutations in at least 30 genes -

Case Report Sarah Burgett, OD Primary Care/Low Vision Resident Marion VAMC Marion, IL
Case Report Sarah Burgett, OD Primary Care/Low Vision Resident Marion VAMC Marion, IL Abstract A 52 year-old African American male has experienced a gradual decrease in visual acuity from 2008 to present. Ocular exams are unremarkable aside from the decrease in visual acuity. Grossly abnormal ERG results confirm progressive cone-rod dystrophy. I. Case History -Patient demographics: 52 year-old African American male -Chief complaint: Vision continues to slowly decline OU. Patient reports increased difficulty reading the Bible and other small print. -Ocular history: Myasthenia gravis x 25 years s/p thymectomy 1985. binding antibody (+), blocking/modulating antibody (-). Presented with ocular symptoms, but has become systemic. Mestinon was tried for his ocular symptoms, but it didn’t help. The patient has been on prednisone since. Doing well on prednisone 10mg QOD x many years. Diplopia/ptosis well controlled at this point. Further tapering (below 5mg/day or 10mg QOD) has been unsuccessful. Patient is follow by Neurology at Marion VA. -Medical history: Hypertension, elevated cholesterol, and pulmonary problems -Medications: 1) Colestipol HCl 1GM TAB to lower cholesterol 2) Diltiazem 180MG SA CAP for heart and blood pressure 3) Fenofibrate 145MG TAB to lower cholesterol and triglycerides 4) Hydrochlorothiazide 25MG TAB for fluid and blood pressure 5) Hydrocodone 5/Acetaminophen 500MG TAB PRN for pain 6) Lisinopril 20MG TAB to lower blood pressure 7) Prednisone 10MG TAB for myasthenia gravis - Allergies: Niacin, Zocor, Pravastatin, Crestor, Zetia 10MG TAB, Lopid 600MG TAB II. Pertinent findings -Clinical: Progressively declining BCVA OU 08/2003 20/20 OD, 20/20 OS 11/2008 20/20 OD, 20/20 OS 04/2009 20/25 OD, 20/30 NIPH OS 06/2010 20/40 OD, 20/50 OS 09/2010 20/30 OD, 20/50-2 12/2010 20/30 OD, 20/60-2 Entrance testing, slit lamp, intraocular pressure, dilated fundus examination all within normal limits OU; no ocular manifestations of myasthenia gravis. -

The Primate Model for Understanding and Restoring Vision
The primate model for understanding and restoring vision Serge Picauda,1, Deniz Dalkaraa,1, Katia Marazovaa, Olivier Goureaua, Botond Roskab,c,d,2, and José-Alain Sahela,e,f,g,2 aInstitut de la Vision, INSERM, CNRS, Sorbonne Université, F-75012 Paris, France; bInstitute of Molecular and Clinical Ophthalmology Basel, CH-4031 Basel, Switzerland; cFaculty of Medicine, University of Basel, 4056 Basel, Switzerland; dFriedrich Miescher Institute, 4058 Basel, Switzerland; eDepartment of Ophthalmology, The University of Pittsburgh School of Medicine, Pittsburgh, PA 15213; fINSERM-Centre d’Investigation Clinique 1423, Centre Hospitalier National d’Ophtalmologie des Quinze-Vingts, F-75012 Paris, France; and gDépartement d’Ophtalmologie, Fondation Ophtalmologique Rothschild, F-75019 Paris, France Edited by Tony Movshon, New York University, New York, NY, and approved October 18, 2019 (received for review July 8, 2019) Retinal degenerative diseases caused by photoreceptor cell death provide highly responsive and reliable models for in-depth are major causes of irreversible vision loss. As only primates have a functional studies. Furthermore, brain-imaging studies de- macula, the nonhuman primate (NHP) models have a crucial role scribing visual cortex activity aid our understanding of the vi- not only in revealing biological mechanisms underlying high-acuity sual system and its functions and also serve as important tools vision but also in the development of therapies. Successful trans- for studying the brain (5–8). lation of basic research findings into clinical trials and, moreover, approval of the first therapies for blinding inherited and age- Uniqueness of the Primate Model for Human Vision related retinal dystrophies has been reported in recent years. -

Progressive Cone and Cone-Rod Dystrophies
Br J Ophthalmol: first published as 10.1136/bjophthalmol-2018-313278 on 24 January 2019. Downloaded from Review Progressive cone and cone-rod dystrophies: clinical features, molecular genetics and prospects for therapy Jasdeep S Gill,1 Michalis Georgiou,1,2 Angelos Kalitzeos,1,2 Anthony T Moore,1,3 Michel Michaelides1,2 ► Additional material is ABSTRact proteins involved in photoreceptor structure, or the published online only. To view Progressive cone and cone-rod dystrophies are a clinically phototransduction cascade. please visit the journal online (http:// dx. doi. org/ 10. 1136/ and genetically heterogeneous group of inherited bjophthalmol- 2018- 313278). retinal diseases characterised by cone photoreceptor PHOTORECEPTION AND THE degeneration, which may be followed by subsequent 1 PHOTOTRANSDUCTION CASCADE UCL Institute of rod photoreceptor loss. These disorders typically present Rod photoreceptors contain rhodopsin phot- Ophthalmology, University with progressive loss of central vision, colour vision College London, London, UK opigment, whereas cone photoreceptors contain 2Moorfields Eye Hospital NHS disturbance and photophobia. Considerable progress one of three types of opsin: S-cone, M-cone or Foundation Trust, London, UK has been made in elucidating the molecular genetics L-cone opsin. Disease-causing sequence variants 3 Ophthalmology Department, and genotype–phenotype correlations associated with in the genes encoding the latter two cone opsins University of California San these dystrophies, with mutations in at least 30 genes -

Novel Compound Heterozygous Mutations Resulting in Cone
Letters seemed to be dark. The narrowing of the retinal vessels and disc 2. Kline LB, Kelly CL. Ocular migraine in a patient with cluster headaches. pallor diminished gradually over time. In the late phase of the Headache. 1980;20(5):253-257. attack (after 1 minute 28 seconds), the retinal vessels were di- 3. Winterkorn JMS, Kupersmith MJ, Wirtschafter JD, Forman S. Brief report: treatment of vasospastic amaurosis fugax with calcium-channel blockers. N Engl lated and the disc was hyperemic. Video 2 and Video 3 show J Med. 1993;329(6):396-398. the reperfusion of the retinal circulation in the late phase. The 4. Bernard GA, Bennett JL. Vasospastic amaurosis fugax. Arch Ophthalmol. images obtained immediately after the attack (Figure 2) show 1999;117(11):1568-1569. the dilated retinal vessels in the right eye. The images show that 5. Hill DL, Daroff RB, Ducros A, Newman NJ, Biousse V. Most cases labeled as the retinal veins and arteries in the right eye were more di- “retinal migraine” are not migraine. J Neuroophthalmol. 2007;27(1):3-8. lated than those in the left eye (not shown) or those obtained 6. Petzold A, Islam N, Plant GT. Video reconstruction of vasospastic transient under normal conditions 1 month after the attack. monocular blindness. N Engl J Med. 2003;348(16):1609-1610. No hypercoagulability was identified with hematologic and serologic testing. Findings on neurologic tests and magnetic Novel Compound Heterozygous Mutations Resulting resonance imaging of the brain were normal. The patient was in Cone Dystrophy With Supernormal Rod Response treated with propranolol hydrochloride because of an allergy Cone dystrophy with supernormal rod response (CDSRR) to lomerizine hydrochloride, and the retinal migraines have (RCD3B, OMIM #610356) was first described in 2 siblings by not recurred. -

Annotation Cone and Cone-Rod Dystrophies
J Med Genet 1992: 29: 289-290 289 Annotation J Med Genet: first published as 10.1136/jmg.29.5.289 on 1 May 1992. Downloaded from Cone and cone-rod dystrophies The retinal dystrophies are a genetically het- examination is usually normal. Two main erogeneous group of disorders which may be forms are recognised. In complete achroma- seen as an isolated ocular abnormality or may topsia (rod monochromatism) there are no be associated with other systemic disease. In functioning cone photoreceptors in the retina.4 most disorders the underlying disease mechan- Vision is reduced to the level of about 6/60, ism is not known, so that classification is un- there is no true colour perception, and there satisfactory. There is great clinical heterogen- are normal rod but absent cone responses on eity even among dystrophies which share a electroretinography. Inheritance is autosomal common mode of inheritance. At present, dys- recessive but there may be more than one trophies are most usefully classified according form. Incomplete achromatopsia or blue cone to whether they are stationary or progressive monochromatism is an X linked recessive dis- and whether there is predominantly macular order which presents in a similar fashion but or generalised retinal disease. The latter group has a better visual prognosis.58 Colour vision is subdivided on the basis of whether there is testing and electroretinography show evidence involvement of rod or cone photoreceptors or of normal rod and blue cone function but both. Most disease is progressive and even- absent red and green cone responses."' tually involves both types of photoreceptors. -
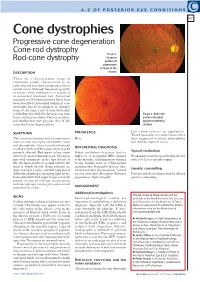
Cone Dystrophies Progressive Cone Degeneration Cone-Rod Dystrophy Figure 1
A-Z OF POSTERIOR EYE CONDITIONS C 47 Cone dystrophies Progressive cone degeneration Cone-rod dystrophy FIGURE 1. Granular Rod-cone dystrophy pattern of pigmentary change at the DESCRIPTION macula These are a heterogenous group of conditions usually characterised by an early, bilateral and slowly progressive loss of central vision. Although frequently sporadic in nature, when inherited, it is usually as an autosomal dominant trait. Autosomal recessive and X-linked recessive forms have been described. Autosomal dominant cone dystrophy has been mapped to chromo- some 17. In some cases of cone dystrophy, rod dysfunction will also develop over time FIGURE 2. Bull’s eye (cone-rod degeneration), whereas in others, pattern of retinal rod dysfunction may precede that of the pigment epithelial cones (rod-cone degeneration). atrophy PREVALENCE Low vision services are appropriate. SYMPTOMS Tinted spectacles or contact lenses have The two most common initial symptoms in Rare. been suggested to reduce photophobia cases of cone dystrophy are blurred vision and thereby improve vision. and photophobia. Once vision has declined to a level of 6/12 to 6/18, colour vision may be DIFFERENTIAL DIAGNOSIS severely affected. Red-green colour vision Other conditions that may lead to Topical medication defects are most commonly seen. Vision loss bull’s-eye or geographic RPE changes Weak miotics may be used during the day may well commence in the first decade of at the maculae, and pigmentary changes such as 0.5 per cent pilocarpine. life, declining slowly to around 6/60 by the in the fundus such as: Chloroquine third or fourth decade. -

Expanding the Clinical, Allelic, and Locus Heterogeneity of Retinal Dystrophies
ORIGINAL RESEARCH ARTICLE © American College of Medical Genetics and Genomics Expanding the clinical, allelic, and locus heterogeneity of retinal dystrophies Nisha Patel, PhD1, Mohammed A. Aldahmesh, PhD1, Hisham Alkuraya, MD2,3, Shamsa Anazi, MSc1, Hadeel Alsharif, BSc1, Arif O. Khan, MD1,4, Asma Sunker, BSc1, Saleh Al-mohsen, MD5, Emad B. Abboud, MD6, Sawsan R. Nowilaty, MD6, Mohammed Alowain, MD7, Hamad Al-Zaidan, MD7, Bandar Al-Saud, MD5, Ali Alasmari, MD8, Ghada M.H. Abdel-Salam, MD9, Mohamed Abouelhoda, PhD1,10, Firdous M. Abdulwahab, BSc1, Niema Ibrahim, BSc1, Ewa Naim, BPharm1,10, Banan Al-Younes, MSc1,10, Abeer E. AlMostafa, BSc1,10, Abdulelah AlIssa, BSc1,10, Mais Hashem, BSc1, Olga Buzovetsky, PhD11, Yong Xiong, PhD11, Dorota Monies, PhD1,10, Nada Altassan, PhD1,10, Ranad Shaheen, PhD1, Selwa A.F. Al-Hazzaa, MD12, and Fowzan S. Alkuraya, MD1,13 Purpose: Retinal dystrophies (RD) are heterogeneous hereditary the likely candidate as AGBL5 and CDH16, respectively. We also disorders of the retina that are usually progressive in nature. The aim performed exome sequencing on negative syndromic RD cases of this study was to clinically and molecularly characterize a large and identified a novel homozygous truncating mutation in GNS in cohort of RD patients. a family with the novel combination of mucopolysaccharidosis and RD. Moreover, we identified a homozygous truncating mutation in Methods: We have developed a next-generation sequencing assay DNAJC17 in a family with an apparently novel syndrome of retinitis that allows known RD genes to be sequenced simultaneously. We also pigmentosa and hypogammaglobulinemia. performed mapping studies and exome sequencing on familial and on syndromic RD patients who tested negative on the panel. -

Keratoconus and Cone-Rod Dystrophy Among Brothers: Clinical Case Study and Genetic Analysis
Trends in Ophthalmology Open Access Journal DOI: 10.32474/TOOAJ.2019.02.000133 ISSN: 2644-1209 Case Report Keratoconus and Cone-Rod Dystrophy among Brothers: Clinical Case Study and Genetic Analysis Victoria Dimacali1*, Spyridon Koronis1, Stavrenia Koukoula1, Achilleas Rasoglou2, Aspasia Adamopoulou1, Elissavet Siskou3 and Miltos Balidis1 1Ophthalmica Institute of Ophthalmology and Microsurgery, Greece 2Department of Ophthalmology, General Hospital of Edessa, Greece 3Department of Ophthalmology, Papanikolaou General Hospital, Greece *Corresponding author: Victoria Dimacali, Ophthalmica Institute of Ophthalmology and Microsurgery, Thessaloniki, Greece Received: March 25, 2019 Published: April 01, 2019 Abstract Background: Advances in genomics continue to enable the discovery of gene variants which cause various inherited ophthalmic disorders. Several case reports have shown an association between keratoconus and retinal disease but whether there is a genetic basis for this is still not known. Methods: Clinical case study with Pentacam imaging, fundus autofluorescence (FAF), macular optical coherence tomography (OCT),Results: electrophysiology We report three studies, brothers, and genetic two of whomanalysis. have keratoconus and one who was found to have bilateral cone-rod dystrophy. MAP3K19, ADGRV1, and PIK3CG This was supported by color vision and electrophysiology testing, fundus autofluorescence,CHST6 gene in and the macular latter. Whole OCT exomefindings. sequencing Genomic data revealedanalysis revealeda rare missense three rare variant gene for variants IMPG2 ( gene in both brothers. ) common to the brother with cone-rod dystrophy and one brother with keratoconus. There was also a very significant variant in the Conclusion: Among the four genes with shared mutations in two of the brothers, IMPG2 has been linked to retinal disease while MAP3K19 and PIK3CG carry high risk scores for keratoconus pathogenesis.