Current Concepts in Dermatology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinicopathological Correlation of Acquired Hyperpigmentary Disorders
Symposium Clinicopathological correlation of acquired Dermatopathology hyperpigmentary disorders Anisha B. Patel, Raj Kubba1, Asha Kubba1 Department of Dermatology, ABSTRACT Oregon Health Sciences University, Portland, Oregon, Acquired pigmentary disorders are group of heterogenous entities that share single, most USA, 1Delhi Dermatology Group, Delhi Dermpath significant, clinical feature, that is, dyspigmentation. Asians and Indians, in particular, are mostly Laboratory, New Delhi, India affected. Although the classic morphologies and common treatment options of these conditions have been reviewed in the global dermatology literature, the value of histpathological evaluation Address for correspondence: has not been thoroughly explored. The importance of accurate diagnosis is emphasized here as Dr. Asha Kubba, the underlying diseases have varying etiologies that need to be addressed in order to effectively 10, Aradhana Enclave, treat the dyspigmentation. In this review, we describe and discuss the utility of histology in the R.K. Puram, Sector‑13, diagnostic work of hyperpigmentary disorders, and how, in many cases, it can lead to targeted New Delhi ‑ 110 066, India. E‑mail: and more effective therapy. We focus on the most common acquired pigmentary disorders [email protected] seen in Indian patients as well as a few uncommon diseases with distinctive histological traits. Facial melanoses, including mimickers of melasma, are thoroughly explored. These diseases include lichen planus pigmentosus, discoid lupus erythematosus, drug‑induced melanoses, hyperpigmentation due to exogenous substances, acanthosis nigricans, and macular amyloidosis. Key words: Facial melanoses, histology of hyperpigmentary disorders and melasma, pigmentary disorders INTRODUCTION focus on the most common acquired hyperpigmentary disorders seen in Indian patients as well as a few Acquired pigmentary disorders are found all over the uncommon diseases with distinctive histological traits. -
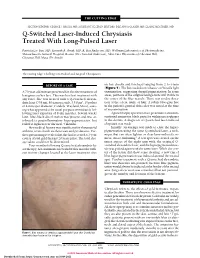
Q-Switched Laser-Induced Chrysiasis Treated with Long-Pulsed Laser
THE CUTTING EDGE SECTION EDITOR: GEORGE J. HRUZA, MD; ASSISTANT SECTION EDITORS: DEE ANNA GLASER, MD; ELAINE SIEGFRIED, MD Q-Switched Laser-Induced Chrysiasis Treated With Long-Pulsed Laser Patricia Lee Yun, MD; Kenneth A. Arndt, MD; R. Rox Anderson, MD; Wellman Laboratories of Photomedicine, Massachusetts General Hospital, Boston (Drs Yun and Anderson), Skin Care Physicians of Chestnut Hill, Chestnut Hill, Mass (Dr Arndt) The Cutting Edge: Challenges in Medical and Surgical Therapeutics REPORT OF A CASE on her cheeks and forehead ranging from 2 to 6 mm (Figure 1). The lesions did not enhance on Wood’s light A 70-year-old woman presented for elective treatment of examination, suggesting dermal pigmentation. In some lentigines on her face. This was her first treatment with areas, portions of the original lentigo were still visible in any laser. She was treated with a Q-switched alexan- the center of the blue macule. There was no discolora- drite laser (755 nm, 50 nanoseconds, 3.5 J/cm2, 15 pulses tion of the sclera, nails, or hair. A subtle blue-gray hue of 4-mm spot diameter; Candela, Wayland, Mass), caus- in the patient’s general skin color was noted at the time ing what appeared to be usual purpura immediately fol- of reexamination. lowing laser exposure of 8 tan macules. Several weeks A punch biopsy specimen of a representative area dem- later, blue-black discoloration was present, and was at- onstrated numerous black particles within macrophages tributed to postinflammatory hyperpigmentation, but in the dermis. A diagnosis of Q-switched laser-induced failed to lighten over the next 4 months. -

Vibratory Urticaria
Vibratory urticaria Description Vibratory urticaria is a condition in which exposing the skin to vibration, repetitive stretching, or friction results in allergy symptoms such as hives (urticaria), swelling ( angioedema), redness (erythema), and itching (pruritus) in the affected area. The reaction can be brought on by towel drying, hand clapping, running, a bumpy ride in a vehicle, or other repetitive stimulation. Headaches, fatigue, faintness, blurry vision, a metallic taste in the mouth, facial flushing, and more widespread swelling (especially of the face) can also occur during these episodes, especially if the stimulation is extreme or prolonged. The reaction occurs within a few minutes of the stimulation and generally lasts up to an hour. Affected individuals can have several episodes per day. Frequency Vibratory urticaria is a rare disorder; its prevalence is unknown. It belongs to a class of disorders called physical urticarias in which allergy symptoms are brought on by direct exposure to factors such as pressure, heat, cold, or sunlight. Physical urticarias have been estimated to occur in up to 5 per 1,000 people. Causes Vibratory urticaria can be caused by a mutation in the ADGRE2 gene. This gene provides instructions for making a protein found in several types of immune system cells, including mast cells. Mast cells, which are found in many body tissues including the skin, are important for the normal protective functions of the immune system. They also play a role in allergic reactions, which occur when the immune system overreacts to stimuli that are not harmful. The specific role of the ADGRE2 protein in mast cells is not well understood. -

Obesity and Chronic Inflammation in Phlebological and Lymphatic Diseases
Review 55 Obesity and chronic inflammation in phlebological and lymphatic diseases G. Faerber Centre for Vascular Medicine, Hamburg Keywords increase in intra-abdominal and intertriginous ten mit venösen oder lymphatischen Erkran- Obesity-associated functional venous insuffi- pressure, which in turn leads to an increase in kungen, die gleichzeitig schwer adipös und ciency, obesity-associated lymphoedema, vis- venous pressure in leg vessels, these relation- häufig multimorbide sind, überproportional ceral obesity, chronic inflammation, insulin ships are mainly caused by the metabolic, an. Die Adipositas, vor allem die viszerale, resistance chronic inflammatory and prothrombotic pro- verschlechtert alle Ödemerkrankungen, er- cesses that result from the increase of visceral höht das Risiko für thromboembolische Er- Summary adipose tissue. These processes can be ident- krankungen und postthrombotisches Syn- The prevalence of obesity has continued to ified by low levels of adiponectin and high lev- drom und kann alleinige Ursache sein für die increase considerably during the past 15 els of leptin, insulin, intact proinsulin, PAI-1 Adipositas-assoziierte funktionelle Venenin- years. Particularly noticeable is the marked and proinflammatory cytokines (IL-6, IL-8, suffizienz ohne Nachweis von Obstruktion increase in morbid obesity, which is in turn TNF-α). Therapeutic measures must therefore oder Reflux. Das Adipositas-assoziierte particularly pronounced among the elderly. be aimed primarily at reducing visceral obesity Lymphödem stellt inzwischen den größten Since the prevalence of venous thromboem- and with it hyperinsulinemia or insulin resis- Anteil unter den sekundären Lymphödemen. bolism, chronic venous insufficiency and sec- tance as well as at fighting chronic inflam- Mehr als 50 Prozent der Lipödempatientin- ondary lymphoedema also increases with mation. -

Urticaria from Wikipedia, the Free Encyclopedia Jump To: Navigation, Search "Hives" Redirects Here
Urticaria From Wikipedia, the free encyclopedia Jump to: navigation, search "Hives" redirects here. For other uses, see Hive. Urticaria Classification and external resourcesICD-10L50.ICD- 9708DiseasesDB13606MedlinePlus000845eMedicineemerg/628 MeSHD014581Urtic aria (or hives) is a skin condition, commonly caused by an allergic reaction, that is characterized by raised red skin wheals (welts). It is also known as nettle rash or uredo. Wheals from urticaria can appear anywhere on the body, including the face, lips, tongue, throat, and ears. The wheals may vary in size from about 5 mm (0.2 inches) in diameter to the size of a dinner plate; they typically itch severely, sting, or burn, and often have a pale border. Urticaria is generally caused by direct contact with an allergenic substance, or an immune response to food or some other allergen, but can also appear for other reasons, notably emotional stress. The rash can be triggered by quite innocent events, such as mere rubbing or exposure to cold. Contents [hide] * 1 Pathophysiology * 2 Differential diagnosis * 3 Types * 4 Related conditions * 5 Treatment and management o 5.1 Histamine antagonists o 5.2 Other o 5.3 Dietary * 6 See also * 7 References * 8 External links [edit] Pathophysiology Allergic urticaria on the shin induced by an antibiotic The skin lesions of urticarial disease are caused by an inflammatory reaction in the skin, causing leakage of capillaries in the dermis, and resulting in an edema which persists until the interstitial fluid is absorbed into the surrounding cells. Urticarial disease is thought to be caused by the release of histamine and other mediators of inflammation (cytokines) from cells in the skin. -
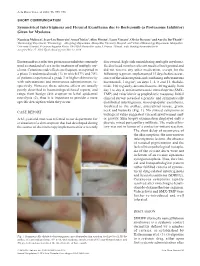
Symmetrical Intertriginous and Flexural Exanthema Due to Bortezomib (A Proteasome Inhibitor) Given for Myeloma
Acta Derm Venereol 2016; 96: 995–996 SHORT COMMUNICATION Symmetrical Intertriginous and Flexural Exanthema due to Bortezomib (a Proteasome Inhibitor) Given for Myeloma Nausicaa Malissen1, Jean-Luc Bourrain1, Anca Chiriac2, Aline Montet1, Laure Vincent3, Olivier Dereure1 and Aurélie Du-Thanh1* 1Dermatology Department, 2Pneumology – Allergology Department, Montpellier University Hospital, and 3Clinical Hematology Department, Montpellier University Hospital, 80 avenue Augustin Fliche, FR-34295 Montpellier cedex 5, France. *E-mail: [email protected] Accepted Mar 17, 2016; Epub ahead of print Mar 22, 2016 Bortezomib is a selective proteasome inhibitor currently discovered, high-risk smouldering multiple myeloma. used as standard of care in the treatment of multiple my- He disclosed no other relevant medical background and eloma. Cutaneous side-effects are frequent, as reported in did not receive any other medication, except for the a phase 3 randomized study (1), in which 57% and 70% following regimen, implemented 15 days before occur- of patients experienced a grade 3 or higher skin toxicity rence of the skin eruption and combining subcutaneous with subcutaneous and intravenous administration, re- bortezomib, 1 mg/m2, on days 1, 4, 8 and 11, thalido- spectively. However, these adverse effects are usually mide, 100 mg daily, dexamethasone, 40 mg daily from poorly described in haematological-based reports, and day 1 to day 4, sulfamethoxazole trimethoprim (SMX- range from benign skin eruption to lethal epidermal TMP) and valaciclovir as prophylactic measures. Initial necrolysis (2), thus it is important to provide a more clinical survey revealed a pruritic and symmetrically specific description when they occur. distributed intertriginous, maculopapular exanthema, localized to the axillae, antecubital fossae, groin, neck and buttocks (Fig. -

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid. -

Module Test № 1 on Dermatology
THE MINISTRY OF HEALTHCARE OF THE RUSSIAN FEDERATION FEDERAL STATE BUDGETARY EDUCATIONAL INSTITUTION OF HIGHER PROFESSIONAL EDUCATION PIROGOV RUSSIAN NATIONAL RESEARCH MEDICAL UNIVERSITY DEPARTMENT OF DERMATOVENEROLOGY Gaydina T.A., Dvornikov A.S., Skripkina P.A., Nazhmutdinova D.K., Heydar S.A., Arutunyan G.B., Pashinyan A.G. MODULE TEST №1 ON DERMATOLOGY FOR STUDENTS OF INSTITUTES OF HIGHER MEDICAL EDUCATION ON SPECIALTY THERAPEUTIC FACULTY DEPARTMENT OF DERMATOVENEROLOGY Moscow 2016 ISBN УДК ББК A21 Module test №1 on Dermatology for students of institutes of high medical education on specialty «Therapeutic faculty» department of dermatovenerology: manual for students for self-training//FSBEI HPE “Pirogov RNRMU” of the ministry of healthcare of the russian federation, M.: (publisher) 2016, 144 p. The manual is a part of teaching-methods on Dermatovenerology. It contains tests on Dermatology on the topics of practical sessions requiring single or multiple choice anser. The manual can be used to develop skills of students during practical sessions. It also can be used in the electronic version at testing for knowledge. The manual is compiled according to FSES on specialty “therapeutic faculty”, working programs on dermatovenerology. The manual is intended for foreign students of 3-4 courses on specialty “therapeutic faculty” and physicians for professional retraining. Authors: Gaydina T.A. – candidate of medical science, assistant of dermatovenerology department of therapeutic faculty Pirogov RNRMU Dvornikov A.S. – M.D., professor of dermatovenerology department of therapeutic faculty Pirogov RNRMU Skripkina P.A. – candidate of medical science, assistant professor of dermatovenerology department of therapeutic faculty Pirogov RNRMU Nazhmutdinova D.K. – candidate of medical science, assistant professor of dermatovenerology department of therapeutic faculty Pirogov RNRMU Heydar S.A. -

Rheumatology Connections
IN THIS ISSUE Tumor Board for Immune-Related Adverse Events 3 | Psoriatic Arthritis Treatment Guidelines 5 | Hypophosphatasia 6 Inflammatory Eye Disease and Hidradenitis Suppurativa 8 | Psoriasis in a Patient with HIV-Related Kaposi Sarcoma 9 Scleredema Adultorum of Buschke 10 | Cutaneous Vasculitis 12 | Leukopenia and Lupus 14 Rheumatology Connections An Update for Physicians | Summer 2019 21581_CCFBCH_19RHE1078_ACG.indd 1 6/6/19 8:52 AM Cleveland Clinic’s Rheumatology Program is ranked among the top From the Chair of Rheumatic and Immunologic Diseases 2 in the nation in U.S. News & World Report’s “America’s Best Dear Colleagues, Hospitals” survey. I am honored to present to you the summer 2019 issue of Rheumatology Connections, an issue I think is particularly representative of the interdisciplinary nature and broad implications of the medicine we practice as rheumatologists. Rheumatology Connections, published by Cleveland Clinic’s Department of Rheumatic and Our frequent collaborations with dermatology underscore the multisystemic nature of our Immunologic Diseases, provides information specialty. Dr. Elaine Husni presents highlights of the first set of collaborative guidelines on on leading-edge diagnostic and management techniques as well as current research for psoriatic arthritis from the American College of Rheumatology and our dermatology colleagues physicians. in the National Psoriasis Foundation (p. 5). Dr. Leonard Calabrese’s ustekinumab case spanning rheumatology, dermatology and oncology is the first of its kind reported (p. 9). Dr. Soumya Please direct any correspondence to: Chatterjee offers a case study on the rare and debilitating scleredema adultorum of Buschke Abby Abelson, MD Chair, Rheumatic and Immunologic Diseases (p. 10). And Dr. -

Etiology, Classification, and Treatment of Urticaria
CONTINUING MEDICAL EDUCATION Etiology, Classification, and Treatment of Urticaria Kjetil Kristoffer Guldbakke, MD; Amor Khachemoune, MD, CWS GOAL To understand urticaria to better manage patients with the condition OBJECTIVES Upon completion of this activity, dermatologists and general practitioners should be able to: 1. Discuss the clinical classification of urticaria. 2. Recognize how to diagnose urticaria. 3. Identify treatment options. CME Test on page 50. This article has been peer reviewed and approved Einstein College of Medicine is accredited by by Michael Fisher, MD, Professor of Medicine, the ACCME to provide continuing medical edu- Albert Einstein College of Medicine. Review date: cation for physicians. December 2006. Albert Einstein College of Medicine designates This activity has been planned and imple- this educational activity for a maximum of 1 AMA mented in accordance with the Essential Areas PRA Category 1 CreditTM. Physicians should only and Policies of the Accreditation Council for claim credit commensurate with the extent of their Continuing Medical Education through the participation in the activity. joint sponsorship of Albert Einstein College of This activity has been planned and produced in Medicine and Quadrant HealthCom, Inc. Albert accordance with ACCME Essentials. Drs. Guldbakke and Khachemoune report no conflict of interest. The authors discuss off-label use of colchi- cine, cyclophosphamide, cyclosporine, dapsone, intravenous immunoglobulin, methotrexate, montelukast sodium, nifedipine, plasmapheresis, rofecoxib, sulfasalazine, tacrolimus, thyroxine, and zafirlukast. Dr. Fisher reports no conflict of interest. Urticaria is among the most common skin dis- autoimmune mechanisms are now recognized as a eases. It can be acute, chronic, mediated by a cause of chronic urticaria. A search of the PubMed physical stimulus, or related to contact with an database (US National Library of Medicine) for urticant. -

Local Heat Urticaria
Volume 23 Number 12 | December 2017 Dermatology Online Journal || Case Presentation DOJ 23 (12): 10 Local heat urticaria Forrest White MD, Gabriela Cobos MD, and Nicholas A Soter MD Affiliations: 1 New York University Langone Health, New York Abstract PHYSICAL EXAMINATION: A brisk, mechanical stroke elicited a linear wheal. Five minutes after exposure We present a 38-year-old woman with local heat to hot water, she developed well-demarcated, urticaria confirmed by heat provocation testing. Heat erythematous blanching wheals that covered the urticaria is a rare form of physical urticaria that is distal forearm and entire hand. triggered by exposure to a heat source, such as hot water or sunlight. Although it is commonly localized Conclusion and immediate, generalized and delayed onset forms Physical or inducible urticarias are a group of exist. Treatment options include antihistamines urticarias that are triggered by various external and heat desensitization. A brisk, mechanical stroke physical stimuli, such as mechanical stimuli, pressure, elicited a linear wheal. Five minutes after exposure cold, light, or temperature change. Urticarias due to hot water, she developed well-demarcated, to temperature change include heat urticaria (HU), erythematous blanching wheals that covered the cholinergic urticaria, and cold urticaria. distal forearm and entire hand. HU is a rare form of chronic inducible urticaria, with Keywords: urticaria, local heat urticaria, physical approximately 60 reported cases [1]. In HU, contact urticaria with a heat source such as hot water, sunlight, hot air, radiant heat, or hot objects results in wheal formation Introduction HISTORY: A 38-year-old woman presented to the Skin and Cancer Unit for the evaluation of recurrent, intensely pruritic eruptions that were precipitated by exposure to heat, which included hot water and sunlight. -

Symmetrical Drug-Related Intertriginous and Flexural Exanthema Secondary to Topical 5-Fluorouracil
Symmetrical Drug-Related Intertriginous and Flexural Exanthema Secondary to Topical 5-Fluorouracil Roxann Powers, MD; Rachel Gordon, MD; Kenrick Roberts, MD; Rodney Kovach, MD We report the case of a 56-year-old man who fossae and axillae. His dermatologist stopped the developed a distinctive skin eruption after treat- 5-FU and started prednisone, erythromycin, hydro- ing actinic keratoses on the dorsal aspects of cortisone ointment, and fexofenadine hydrochloride. his right and left hands with topical 5-fluorouracil Over the next week, the areas became eroded and the (5-FU). The distribution of his rash was charac- pain worsened. A biopsy was performed. The hydro- teristic of symmetrical drug-related intertriginous cortisone was discontinued and he was referred for a and flexural exanthema (SDRIFE), also known as second opinion. baboon syndrome. Medications at presentation included erythromycin, Cutis. 2012;89:225-228. fexofenadine hydrochloride, acetaminophen-codeine, CUTISprednisone, petrolatum ointment, metformin Case Report hydrochloride, enalapril maleate, ranitidine hydro- A 56-year-old man with a history of diabetes mel- chloride, simvastatin, triamterene/hydrochlorothiazide, litus, hypertension, hyperlipidemia, reflux, squamous aspirin, and omeprazole. He reported no cell carcinoma in situ of his right hand, and actinic known allergies. keratoses of the right Doand left hands presentedNot with a PhysicalCopy examination revealed an overweight painful, burning, pruritic, erythematous, and blister- man who appeared agitated and reported substantial ing rash on his medial thighs, scrotum, penis, antecu- discomfort. He had well-demarcated, violaceous, red bital fossae, axillae, and dorsal hands. The patient had erosions on his medial thighs (Figure 1), scrotum, a successful history of treatment of actinic keratosis and penis; erythematous denuded patches in the on his right hand with topical 5-fluorouracil (5-FU) antecubital fossae (Figure 2) and axillae; and crusty 8 years prior to presentation.