Supplementary Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bitamoazedthesis.Pdf (2.274Mb)
GASTROINTESTINAL ABSORPTION OF HEPARINS A Dissertation Submitted to the College of Graduate Studies and Research in Partial Fulfillments of the Requirements for the Degree of Doctor of Philosophy in the Graduate Program of Veterinary Biomedical Sciences University of Saskatchewan Saskatoon Bita Moazed Copyright Bita Moazed, November 2009. All Rights Reserved PERMISSION TO USE In presenting this dissertation in partial fulfillment of the requirements for a Doctor of Philosophy degree from the University of Saskatchewan, I agree that the Libraries of this University may make it freely available for inspection. I further agree that permission for copying of this thesis/dissertation in any manner, in whole or in part, for scholarly purposes may be granted by Dr. Linda M. Hiebert who supervised my dissertation work or, in her absence, by the chair of the Graduate Program of Veterinary Biomedical Sciences. It is understood that any copying or publication or use of this dissertation or parts thereof for financial gain shall not be allowed without my written permission. It is also understood that due recognition shall be given to me and the University of Saskatchewan in any scholarly use which may be made of any material in this dissertation. Requests for permission to copy or to make other use of other material in this dissertation in whole or part should be addressed to: Chair of the Graduate Program of Veterinary Biomedical Sciences Western College of Veterinary Medicine University of Saskatchewan 52 Campus Drive Saskatoon, Saskatchewan S7N 5B4 Canada i PREFACE This dissertation has been organized as a series of manuscripts that was or will be submitted for publication in scientific journals. -

Presentación De Powerpoint
A FAVORABLE DECREASE IN THROMBOCYTE LEVELS CAUSED BY LMWH TREATMENT FOR A DVT PATIENT WITH REFRACTER ESSENTIAL THROMBOCYTOSIS:A case report Hakan Keski, MD, Hematology University of Health Sciences Ümraniye Educaon and Research Hospital, Clinic of Hematology Introducon: Low molecular weightheparins have been found at least as eecve as unfraconed heparin in proximal venous thrombosis and have been shown to further inhibit the in vivo thrombin generaon. Because of the various reasons, there may be some changes among the LMWHs regarding the paent’sclinical follow-ups. Case report: A 53-year-old male paent, paent weight: 88 kg For the paent JAK2 V617F posive essenal thrombocytosis (ET) was diagnosed in Haydarpaa Numune Educaon and Research Hospital in 2006. Paent has been followed in Ümraniye Educaon and Research Hospital the hematology clinic since May 2016. Medical Story (anamnesis): One month nadroparin calcium 3800 IU treatment was given to paent due to lower extremity deep vein thrombosis (DVT) in June 2008.The paent had DVT for the second me in the lower extremity in September 2013, this me enoxaparin sodium 6000 IU was given as treatment. Physical examinaon: Splenomegaly was conrmed with palpaon for 2 cm (168 mm measured with USG) Laboratory: At the me of diagnosis, WBC 10880, Hgb: 15,9 g/dl Hct % 46 Plt: 1820000, biochemical parameters such as urea, creanine, AST, ALT were normal. The paent inially received ASA 100 mg and hydroxyurea 500 mg 3x1 was also given and 3 months later, platelet value was 1750000, the paent has been accepted as refractor and anagrelide treatment has been added with the dossage 2x1, then 3x1 in addion to the current treatment. -

Thromboprophylaxis for Venous Thromboembolism UHL Guideline
Guidelines for Pharmacological and Mechanical Thromboprophylaxis for venous thromboembolism. Approved By: Policy and Guideline Committee Date Approved: 12 February 2016 Trust Reference: B9/2016 Version: V4 – 16 August 2019 Policy and Guideline Committee Supersedes: V3 February 2016 Author / Originator(s): Simon Rudge, Venous Thrombosis Nurse Name of Responsible Thrombosis Prevention Committee Committee/Individual: Review Date: August 2022 CONTENTS Section Page 1. Introduction 3 2. Policy Scope 5 VTE Risk Assessment and Pharmacological and Mechanical venous 3. 6 thromboprophylaxis 4. Patient information 8 5. Mechanical thromboprophylaxis. Application and management guide 9 6. Nursing care 12 7. References 12 8. Legal Liability Guideline Statement 12 Appendix 1 Derogation from NICE NG89: VTE risk assessment of 16 & 17yr olds 13 Appendix 2a/2a1/2b/2c VTE risk assessment tools 14-18 Appendix 3 NICE CG92 algorithm for VTE thromboprophylaxis in medical patients 19 Appendix 4 Consensus of risk factors for VTE in surgical patients 20 Appendix 5 UHL and East Midlands approved List of cohort Day Case procedures 21 Appendix 6 Thromboprophylaxis administration guide: Dalteparin 22 Appendix 7 Derogation from NICE NG89: minimum of 7 days low molecular weight 23 heparin for acutely unwell medical patients Appendix 8 Indications for mechanical thromboprophylaxis algorithm 24 Appendix 8a Indications for mechanical thromboprophylaxis with intermittent 25 pneumatic compression devices algorithm Appendix 9 Quick reference guide of NICE NG89 26 Summary of key changes: Addition of procedure specific recommendations from NG89. Inclusion of discharge recommendations. Inclusion of statement regarding medicines of animal origin. Inclusion of training requirements Inclusion of statements of derogation from NG89 Addition of quick reference guide of NG89. -

Review of Anticoagulant Drugs in Paediatric Thromboembolic Disease
18th Expert Committee on the Selection and Use of Essential Medicines (21 to 25 March 2011) Section 12: Cardiovascular medicines 12.5 Antithrombotic medicines Review of Anticoagulant Drugs in Paediatric Thromboembolic Disease September 2010 Prepared by: Fiona Newall Anticoagulation Nurse Manager/ Senior Research Fellow Royal Children’s Hospital/ The University of Melbourne Melbourne, Australia 1 Contents Intent of Review Review of indications for antithrombotic therapy in children Venous thromboembolism Arterial thrombeombolism Anticoagulant Drugs Unfractionated heparin Mechanism of action and pharmacology Dosing and administration Monitoring Adverse events Formulary Summary recommendations Vitamin K antagonists Mechanism of action and pharmacology Dosing and administration Monitoring Adverse events Formulary Summary recommendations Low Molecular Weight Heparins Mechanism of action and pharmacology Dosing and administration Monitoring Adverse events Formulary Summary recommendations ??Novel anticoagulants Summary 2 1. Intent of Review To review the indications for anticoagulant therapies in children. To review the epidemiology of thromboembolic events in children. To review the literature and collate the evidence regarding dosing, administration and monitoring of anticoagulant therapies in childhood. To review the safety of anticoagulant therapies and supervision required To give recommendations for the inclusion of anticoagulants on the WHO Essential Medicines List 2. Review of indications for antithrombotic therapy in children Anticoagulant therapies are given for the prevention or treatment of venous and/or arterial thrombosis. The process of ‘development haemostasis’, whereby the proteins involved in coagulation change quantitatively and qualitatively with age across childhood, essentially protects children against thrombosis(1-5). For this reason, insults such as immobilization are unlikely to trigger the development of thrombotic diseases in children. -

Hemosil ® Liquid Anti-Xa
H E M O S I L® LIQUID ANTI-XA Measuring heparin and apixaban: Simple, fast, 24/7 • Liquid formulation, ready-to-use • One-stage, chromogenic anti-Xa assay • Universal calibration for unfractionated heparin (UFH) and low molecular weight heparin (LMWH) • Drug specific calibrators and controls for measurement of apixaban Measuring heparin and apixaban Unfractionated and low molecular weight heparin Heparin is a highly sulfated polysaccharide Laboratory monitoring is extremely important to characterized by a wide molecular weight range and assess the appropriate level of anticoagulation in potent anticoagulant activity. It exists either as UFH patients receiving UFH. Anti-Xa is recommended for or as depolymerized LMWH. UFH and LMWH have measuring both UFH and LMWH. a rapid anticoagulant effect and are used in the prevention and treatment of venous thrombosis and Anti-Xa testing for measuring UFH helps improve acute coronary syndrome. quality of care and patient experience while reducing costs, when compared with APTT testing.1 UFH and LMWH anticoagulant activity occurs when The advantages include: a complex with antithrombin (AT) is formed, • Higher precision potentiating its anticoagulant activity up to • Lower levels of discordant results1,2,4 1,000-fold, which inactivates both thrombin (FIIa) • Faster time to achieve therapeutic levels1,3,4 and Factor Xa (FXa). UFH acts through both FIIa 1,3,4,5 and FXa inhibition, while LMWH is a more efficient • Fewer tests and dosage changes catalyst for FXa inhibition. Direct Xa inhibitors Anticoagulation for patients with venous DOACs do not require routine monitoring. However, thromboembolism (VTE) previously included there are specific instances when an understanding heparin, heparin derivatives and/or oral vitamin K of the DOAC concentration in a patient sample may antagonists. -

The Management of Anticoagulants Using the Chart: – Low Molecular Weight Heparins (I.E
WA Adult Anticoagulation Medication Chart Overview This presentation will provide an overview of: • The layout of the WA Anticoagulation Medication Chart (WA AMC) • The management of anticoagulants using the chart: – Low Molecular Weight Heparins (i.e. enoxaparin) – Unfractionated heparin (UFH) – Warfarin – Direct oral anticoagulants (DOACs) Anticoagulants – High Risk Medications • Anticoagulants are consistently identified as causing preventable harm to patients. • Top 20 medications involved in medication errors (July 2016 - June 2017) 1. Paracetamol 11. Buprenorphine 2. Enoxaparin 12. Targin (Oxycodone / naloxone) 3. Novorapid Insulin 13. Warfarin 4. Tramadol 14. Diazepam 5. Heparin 15. Tapentadol 6. Fentanyl 16. Metformin 7. Piperacillin & Tazobactam 17. Clonazepam 8. Oxycodone 18. Frusemide 9. Lantus Insulin 19. Hydromorphone 10. Vancomycin 20. Quetiapine • When used in error or omitted, they can cause life-threatening or fatal bleeding or thrombosis. Those most commonly prescribed anticoagulants are: –unfractionated heparin –low-molecular weight heparin (LMWH) • enoxaparin sodium (Clexane®) • dalteparin sodium (Fragmin®) and – warfarin. Direct oral anticoagulants are also available and are being prescribed more frequently: –dabigatran (Pradaxa®) –rivaroxaban (Xarelto®) –apixaban (Eliquis®). Factors that increase the potential for error and harm include: • Low margin for error – over-dose → bleeding – under-dose or omission → thrombosis • Wide variation in individual patient response – multiple indications – wide range and complexity of dosage – frequent dose adjustment/monitoring – interaction with other medicines, herbals, over-the-counter products, food and alcohol. Benefits of the WA Anticoagulant Medication Chart • Provides one chart for all anticoagulant prescriptions to reduce the risk of duplicate prescribing. • Point of care guidelines for initiation, monitoring and reversal of anticoagulants. • Enables the effective achievement of therapeutic levels. -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -
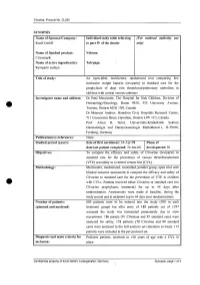
For National Autllority Use Only
Clivarine. Protocol No. CL055 SYNOPSIS Name of Sponsor/Company: Individual study table referring (For national autllOrity use Knoll GmbH to part IV of the dossier only) Name of finished product: Volume: Clivarine® Name of active ingredient(s): Tab/page: Reviparin sodium Title of study: An open-label, multicentre, randomized trial comparing low molecular weight heparin (reviparin) to standard care for the prophylaxis of deep vein thrombosis/pulmonary embolism in children with central venous catheters Investigator name and address: Dr Patti Massicotte, The Hospital for Sick Children, Division of Hematology/Oncology, Room 9420, 555 University Avenue, Toronto, Ontario M5G JX8, Canada Dr Maureen Andrew, Hamilton Civic Hospitals Research Center, 711 Concession Street, Hamilton, Ontario L8V JC3, Canada Prof. Anton H. Sutor, Universitats-Kinderklink Sektion Haematologie und Haemostaseologie Mathildenstr.l, D-79106, Freiburg, Germany Publication(s) (reference): None. Studied period (years): date of first enrolment: 24-Apr-98 Phase of date' last patient completed: 24-Jan-00 development: 111 Objectives: To compare the efficacy and safety of Clivarine (reviparin) to standard care for the prevention of venous thromboembolism (VTE) secondary to a central venous line (CVL). Methodology: Multicentre, randomized, controlled, parallel group, open trial with blinded outcome assessment to compare the efficacy and safety of Clivarine to standard care for the prevention of VTE in children e· with CVLs. Patients received either Clivarine or standard care (no -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Adverse Drug Reactions in Older Adults
Dubrall et al. BMC Pharmacology and Toxicology (2020) 21:25 https://doi.org/10.1186/s40360-020-0392-9 RESEARCH ARTICLE Open Access Adverse drug reactions in older adults: a retrospective comparative analysis of spontaneous reports to the German Federal Institute for Drugs and Medical Devices Diana Dubrall1,2* , Katja S. Just3, Matthias Schmid1, Julia C. Stingl3 and Bernhardt Sachs2,4 Abstract Background: Older adults are more prone to develop adverse drug reactions (ADRs) since they exhibit numerous risk factors. The first aim was to analyse the number of spontaneous ADR reports regarding older adults (> 65) in the ADR database of the German Federal Institute for Drugs and Medical Devices (BfArM) and to set them in relation to i) the number of ADR reports concerning younger adults (19–65), and ii) the number of inhabitants and assumed drug-exposed inhabitants. The second aim was to analyse, if reported characteristics occurred more often in older vs. younger adults. Methods: All spontaneous ADR reports involving older or younger adults within the period 01/01/2000–10/31/2017 were identified in the ADR database. Ratios concerning the number of ADR reports/number of inhabitants and ADR reports/drug-exposed inhabitants were calculated. The reports for older (n = 69,914) and younger adults (n = 111, 463) were compared using descriptive and inferential statistics. Results: The absolute number of ADR reports involving older adults increased from 1615 (2000) up to 5367 ADR reports (2016). The age groups 76–84 and 70–79 had the highest number of ADR reports with 25 ADR reports per 100,000 inhabitants and 27 ADR reports per 100,000 assumed drug-exposed inhabitants. -

Dalteparin Sodium Injection) in Children with Venous Thromboembolism with Or Without Malignancies
FRAG-A001-201 (A6301094) Final Protocol Amendment 9, 18 October 2016 CLINICAL STUDY PROTOCOL A THREE MONTH PROSPECTIVE OPEN LABEL STUDY OF THERAPY WITH FRAGMIN® (DALTEPARIN SODIUM INJECTION) IN CHILDREN WITH VENOUS THROMBOEMBOLISM WITH OR WITHOUT MALIGNANCIES Compound: PN180524 Compound Name : Dalteparin Sodium Injection (Fragmin®) United States (US) Investigational New IND 79,617 Drug (IND) Number: European Clinical Trials Database 2016-000394-21 (EudraCT) #: Protocol Number: FRAG-A001-201 (A6301094) Phase: II Version #: Amendment 9 – 18 October 2016 Pfizer Inc 235 East 42nd Street New York, NY 10017 - Do Not Distribute Page 1 FRAG-A001-201 (A6301094) Final Protocol Amendment 9, 18 October 2016 Document History This amendment incorporates all revisions to date, including amendments made at the request of country health authorities, institutional review boards/ethics committees (IRBs/ECs), etc. Document Date Summary of Changes and Rationale Original Protocol June 23, 2008 Legacy Eisai Inc Protocol Protocol Amendment 1 September 04, 2008 Legacy Eisai Inc Protocol Correction to Amendment 1 January 05, 2009 Legacy Eisai Inc Protocol Protocol Amendment 2 February 20, 2009 Legacy Eisai Inc Protocol Protocol Amendment 3 September 15, 2010 Legacy Eisai Inc Protocol Protocol Amendment 4 September 01, 2011 Legacy Eisai Inc Protocol Protocol Amendment 5 April 21, 2015 Administrative changes per transition of study to Pfizer Inc from Eisai; Updating of Safety Section per Pfizer safety reporting processes and procedures, and other relevant sections per Pfizer Inc processes and procedures. Protocol Amendment 6 09 September 2015 This version was never finalized or submitted and was replaced by Amendment 7. Protocol Amendment 7 18 November 2015 Includes protocol modifications endorsed by FDA in a Type C Meeting conducted on 05 November 2015, including updating age cohort groups, inclusion of all patients with VTE and removal of the central imaging reader and Adjudication Committee. -

Anderson Chapt 8
Chapter-8 Bibliography Where is the evidence? Literature Cited Alpert JS, Dalen JE. Epidemiology and natural history of venous thromboembo- lism. Prog Cardiovas Dis 1994; 36:417-22. Anderson FA, Wheeler HB, Golberg RJ, Hosmer DW, Forcier A, Patwardhan NA. Changing clinical practice: Prospective study of the impact of continuing medical education and quality assurance programs on use of prophylaxis for venous thromboembolism Arch Intern Med 1994; 154:669-677. Anderson FA Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Forcier A, Patwardhan NA. Physician practices in the prevention of venous thromboembolism. Ann Intern Med 1991;115:591-595. Anderson FA Jr, Wheeler HB. Strategies to improve implementation. In: Goldhaber S, ed. Prevention of Venous Thromboembolism. New York, NY: Marcel Dekker Inc.; 1992; 519-539. Bergqvist D. 1983. Post-operative Thromboembolism, Frequency, Etiology, Prophylaxis. Berlin: Springer-Verlag. Clagett GP, Anderson FA Jr, Heit J, Levine MN, Wheeler HB. Prevention of venous thromboembolism. Chest 1995; 108:312S-334S. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. N Engl J Med 1988; 318:1162-73. Gallus AS. Anticoagulants in the prevention of venous thromboembolism. Baillieres Clin Haematol 1990; 3(3):651-684. Hull RD, Raskob GE, Hirsh J. The diagnosis of clinically suspected pulmonary embolism: Practical approaches. Chest 1986; 89(5 Suppl):417S-425S. Morrell MP, Dunhill MA. The postmortem incidence of pulmonary embolism in a hospital population. Br J Surg 1968; 55:347-352. NIH Consensus Development. Prevention of venous thrombosis and pulmonary Best embolism. JAMA 1986; 256:744-749.