An Experimental Investigation of Coloring Matter in Flowers of Vinca Major
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparison of the Production of Polyphenol Contents and the Expression of Genes Involved in Vietnamese Tea Cultivars
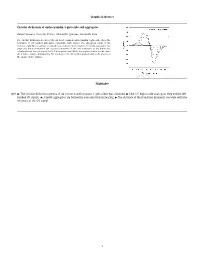
International Food Research Journal 26(6): 1781-1788 (December 2019) Journal homepage: http://www.ifrj.upm.edu.my A comparison of the production of polyphenol contents and the expression of genes involved in Vietnamese tea cultivars 1Hoang, T. T. Y., 2Luu, H. L., 2Nguyen, T. L., 3Duong, T. D., 4,5Nguyen, H. D. and 2*Huynh, T. T. H 1Thai Nguyen University of Sciences, Thai Nguyen University, Thai Nguyen Province 24000, Vietnam 2Institute of Genome Research, Vietnam Academy of Science and Technology (VAST), Hanoi 100000, Vietnam 3Thai Nguyen University of Agriculture and Forestry, Thai Nguyen University, Thai Nguyen Province 24000, Vietnam 4Advanced Centre for Bioorganic Chemistry, Institute of Marine Biochemistry, VAST, Hanoi 100000, Vietnam 5University of Science and Technology of Hanoi, VAST, Hanoi 100000, Vietnam Article history Abstract Received: 19 June, 2019 Tea (Camellia sinensis) is a popular health beverage which is consumed all over the world Received in revised form: due to its good aroma and taste. Tea consumption is also considered to reduce the risk of 16 September, 2019 several diseases in humans, including cardiovascular diseases, diabetes and cancers. Recent Accepted: 25 September, 2019 studies have shown that polyphenols derived from tea may contribute to the majority of these pharmaceutical properties. Among all the tea polyphenols, catechins are the main components that include (−)-epicatechin (EC), (−)-epicatechin gallate (ECG), (−)-epigallocatechin (EGC), (−)-epigallocatechin-3 gallate (EGCG), (+)-catechin (C), (−)-catechin gallate (CG), (−)-gallocatechin (GC), and (−)-gallocatechingallate (GCG). In the present work, four Keywords catechins (C, EGC, ECG, and EGCG) and two anthocyanidins (cyanidin 3-O-glucoside and delphinidin 3-O-glucoside) in two Vietnamese tea cultivars, Trungduxanh and Trungdutim, were Catechin LAR quantitatively detected by high-performance liquid chromatography. -
Graphical Abstract Circular Dichroism of Anthocyanidin 3-Glucoside Self
Graphical abstract Circular dichroism of anthocyanidin 3-glucoside self-aggregates Raquel Gavara, Vesselin Petrov, Alexandre Quintas, Fernando Pina * The circular dichroism spectra of the six most common anthocyanidin 3-glucoside show the formation of left handed aggregates compatible with dimers. The absorption bands of the monomer split by increasing concentration according to the formation of H and J aggregates. The angle and distance between the transition moments of the two monomers in the dimer was calculated from the splitting of the 0–0 absorption band. While the angle is similar for the series the distance changes dramatically. The intensity of the CD signal is proportional to the inverse of the square of the distance. Highlights Q10 " The circular dichroism spectra of six common anthocyanins 3-glucosides was obtained. " Like 3,5-diglucoside analogous they exhibit left- handed CD signals. " J and H aggregates are formed by concentration increasing. " The distance of the transition moments correlate with the intensity of the CD signal. 1 1 2 Circular dichroism of anthocyanidin 3-glucoside self-aggregates a a b a,⇑ 3 Q1 Raquel Gavara , Vesselin Petrov , Alexandre Quintas , Fernando Pina 4 a REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829516 Monte de Caparica, Portugal 5 b Instituto Superior de Ciencias da Saude Egas Moniz, Centro de Investigação Interdisciplinar Egas Moniz, P-2829511 Monte de Caparica, Caparica, Portugal 6 7 article info a b s t r a c t 1 9 10 Self-association constants for the flavylium cations of the six most common anthocyanidin 3-glucosides 20 11 were determined by circular dichroism (CD) and UV–Vis spectroscopy. -

Electrochemical and Antioxidant Properties of Anthocyanins and Anthocyanidins
CROATICA CHEMICA ACTA CCACAA 80 (1) 29¿34 (2007) ISSN-0011-1643 CCA-3135 Original Scientific Paper Electrochemical and Antioxidant Properties of Anthocyanins and Anthocyanidins Andréia A. de Lima, Eliana M. Sussuchi, and Wagner F. De Giovani* Departamento de Química, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Av. Bandeirantes 3900, 14040-901, Ribeirão Preto – SP, Brazil RECEIVED JUNE 26, 2006; REVISED SEPTEMBER 29, 2006; ACCEPTED OCTOBER 27, 2006 Electrochemical properties of delphinidin, cyanidin, pelargonidin, kuromanin and callistephin were investigated by cyclic and differential pulse voltammetries at different pH values and also in methanol. On the basis of oxidation potentials, the order of antioxidant activity for antho- cyanidins is delphinidin > cyanidin > pelargonidin. Oxidation peaks for anthocyanins (kuro- manin and callistephin) are shifted to more positive potentials compared to anthocyanidins (delphinidin, cyanidin and pelargonidin). Oxidation peak currents are linearly dependent on the Keywords square root of the scan rate, which is typical of a diffusion controlled electrochemical process. anthocyanins Antioxidant activities of the compounds were evaluated using the 1,1-diphenyl-2-picrylhydra- anthocyanidins zyl (DPPH) radical-scavenging method and they are directly related to their redox potential antioxidants values. The order of the antioxidant activity is delphinidin > cyanidin > pelargonidin > kuro- electrochemical properties manin > callistephin. INTRODUCTION pharmacological properties that render them interesting potential cancer chemopreventive agents.9–11 Because Anthocyanins are one of the main classes of flavonoids anthocyanins are widely consumed, finding out addi- in red wines. They have been pointed out as the molecu- tional biological activities related to these compounds lar entities that are most likely responsible for what has would be of great interest. -

RP HPLC Determination of Some Uncommon Anthocyanins
360 УДК 543.54:547.814.5 RP HPLC determination of some uncommon anthocyanins Deineka V.I., Vu Thi Ngoc Anh, Deineka L.A. Federal State Autonomous Educational Institution of Higher Professional Education «Belgorod National Research University» RF, Belgorod Received 12.02.2014 Abstract Anthocyanins of Catharanthus roseus petals were found to be composed of pairs (3-galactosides and 3-rhamnosylgalactosides) mainly of 7-methylated anthocyanidins of “dephinidin series” (7-methyldelphinidin, 7-methylpetunidin and hirsutidin); though the same derivatives of “cyanidin series” are present in low concentrations. Flowers of Caesalpinia pulcherrima contain two 3-glucosides: of cyanidin and 5-methylcyanidin. The migration of CH 3-radical from 3’-OH group to 7-OH position results in increase of retention, while for the migration to 5-OH group a decrease of retention was observed. Different position of ring A OH-groups methylation can be predicted also by controversial shift of λmax of the solutes. Keywords: Reversed-phase HPLC, uncommon anthocyanins, 7-OH methylation; 5-OH methylation; regularities of retention alteration, electronic spectra . Установлено , что антоцианы лепестков цветков Catharanthus roseus образованы парами (3-галактозидами и 3-рамнозилгалактазидами ) в-основном антоцианидинами дельфинидинового ряда с метилированием OH-группы в положении 7: 7-метилдельфинидином , 7-метилпетунидином и 7- метилмальвидином ( хирсутидином ); впрочем , аналогичные производные антоцианов цианидинового ряда также присутствуют , но в небольших концентрациях . Антоцианы высушенных цветков Caesalpiniapulcherrima содержат два 3-глюкозида : цианидина и 5-метилцианидина . Перемещение CH 3-радикала из 3’-OH группы к 7-OH группе сказывается в заметном увеличении удерживания , но при аналогичном переносе на ОН -группу в положение 5 наблюдается уменьшение удерживания . Различный тип метилирования ОН -групп кольца А приводит к противоположным смещениям λmax антоцианов . -

Solutions That Meet Your Demands for Food Testing & Agriculture
Solutions that meet your demands for food testing & agriculture Our measure is your success. Excellent choices for food & agriculture applications products I applications I software I services Agilent Technologies Consumer Products Toys, jewelry, clothing, and other products are frequently recalled due to the presence of unsafe levels of substances such as lead from paint and phthal- ates from product polymers and packaging. Whether your perspective is to guarantee your products are free of contaminants or you are screening for harmful contaminants in a wide variety of consumer products, Agilent Tech- nologies provides the tools you need to detect and measure these and other harmful contaminants. > Search entire document Agilent 1290 Infinity LC with Agilent Poroshell columns for simultaneous determination of eight organic UV filters in under two minutes Application Note Consumer Products Authors Siji Joseph Agilent Technologies India Pvt. Ltd. mAU Amino benzoic acid Bangalore, India 2 Oxybenzone 1.5 4-Methyl benzylidene camphor Dioxybenzone Avobenzone Michael Woodman 1 Octyl methoxycinnamate 0.5 Octocrylene Agilent Technologies, Inc. Octyl salicylate 2850 Centerville Road 0 0 0.5 1 1.5 2 min Wilmington DE 19808 USA Abstract Levels of UV filters in personal care products are regulated by the FDA and European Pharmacopeia (EP). Liquid chromatographic (LC) methods are widely accepted analyt- ical techniques for the qualitative and quantitative analysis of these UV filters. Most of these traditional LC methods require about 25–50 minutes. In this Application Note, the Agilent 1290 Infinity LC, in combination with Agilent Poroshell columns, were used for development of a short, sensitive, robust and well resolved separation of eight FDA/EP approved active UV filter ingredients in 99 seconds. -

L.) Leaves Tao Jiang1,3, Kunyuan Guo2,3, Lingdi Liu1, Wei Tian1, Xiaoliang Xie1, Saiqun Wen1 & Chunxiu Wen1*
www.nature.com/scientificreports OPEN Integrated transcriptomic and metabolomic data reveal the favonoid biosynthesis metabolic pathway in Perilla frutescens (L.) leaves Tao Jiang1,3, Kunyuan Guo2,3, Lingdi Liu1, Wei Tian1, Xiaoliang Xie1, Saiqun Wen1 & Chunxiu Wen1* Perilla frutescens (L.) is an important medicinal and edible plant in China with nutritional and medical uses. The extract from leaves of Perilla frutescens contains favonoids and volatile oils, which are mainly used in traditional Chinese medicine. In this study, we analyzed the transcriptomic and metabolomic data of the leaves of two Perilla frutescens varieties: JIZI 1 and JIZI 2. A total of 9277 diferentially expressed genes and 223 favonoid metabolites were identifed in these varieties. Chrysoeriol, apigenin, malvidin, cyanidin, kaempferol, and their derivatives were abundant in the leaves of Perilla frutescens, which were more than 70% of total favonoid contents. A total of 77 unigenes encoding 15 enzymes were identifed as candidate genes involved in favonoid biosynthesis in the leaves of Perilla frutescens. High expression of the CHS gene enhances the accumulation of favonoids in the leaves of Perilla frutescens. Our results provide valuable information on the favonoid metabolites and candidate genes involved in the favonoid biosynthesis pathways in the leaves of Perilla frutescens. Perilla frutescens (L.), which is a self-compatible annual herb, belongs to the family Lamiaceae. Tis species has been widely cultivated in China, Japan, and Korea for centuries. Perilla frutescens is an important medicinal and edible plant in China with medical and nutritional uses 1. Its leaves can be utilized as a transitional medicinal herb, as a vegetable, and as a spice, and its seeds can be processed into foods and nutritional edible oils 2. -

What Pigments Are in Plants?
BUILD YOUR FUTURE! ANYANG BEST COMPLETE MACHINERY ENGINEERING CO.,LTD WHAT PIGMENTS ARE IN PLANTS? Pigments Pigments are chemical compounds responsible for color in a range of living substances and in the inorganic world. Pigments absorb some of the light they receive, and so reflect only certain wavelengths of visible light. This makes them appear "colorful.” Cave paintings by early man show the early use of pigments, in a limited range from straw color to reddish brown and black. These colors occurred naturally in charcoals, and in mineral oxides such as chalk and ochre. The WebExhibit on Pigments has more information on these early painting palettes. Many early artists used natural pigments, but nowadays they have been replaced by cheaper and less toxic synthetic pigments. Biological Pigments Pigments are responsible for many of the beautiful colors we see in the plant world. Dyes have often been made from both animal sources and plant extracts . Some of the pigments found in animals have also recently been found in plants. Website: www.bestextractionmachine.com Email: [email protected] Tel: +86 372 5965148 Fax: +86 372 5951936 Mobile: ++86 8937276399 BUILD YOUR FUTURE! ANYANG BEST COMPLETE MACHINERY ENGINEERING CO.,LTD Major Plant Pigments White Bird Of Paradise Tree Bilirubin is responsible for the yellow color seen in jaundice sufferers and bruises, and is created when hemoglobin (the pigment that makes blood red) is broken down. Recently this pigment has also been found in plants, specifically in the orange fuzz on seeds of the white Bird of Paradise tree. The bilirubin in plants doesn’t come from breaking down hemoglobin. -

Callistephin Enhances the Protective Effects of Isoflurane on Microglial Injury Through Downregulation of Inflammation and Apoptosis
802 MOLECULAR MEDICINE REPORTS 20: 802-812, 2019 Callistephin enhances the protective effects of isoflurane on microglial injury through downregulation of inflammation and apoptosis LILI ZHAO, SHIBIAO CHEN, TIANYIN LIU, XIUHONG WANG, HAIJIN HUANG and WEICHENG LIU Department of Anesthesiology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi 330006, P.R. China Received June 18, 2018; Accepted March 15, 2019 DOI: 10.3892/mmr.2019.10282 Abstract. Microglia are the major immune cells in the central enhanced the effects of isoflurane. Callistephin may therefore nervous system. Microglial activation can be beneficial or constitute a candidate drug agent that may target inflammatory detrimental depending on the stimuli and the physiopathological and growth regulatory signaling pathways, thus ameliorating environment. Microglial activation is involved in a variety certain aspects of neurodegenerative diseases. of neurodegenerative disorders. Different anesthetic agents have exhibited diverse effects on microglial activation and Introduction the engulfment process. The anthocyanin callistephin has been demonstrated to have antioxidant and anti‑inflammatory Microglial cells are the major immune cell in the central properties, and these were assessed in the present study, with a nervous system (CNS), responding against types of endog- focus on its effect on microglial activation. Mouse microglial enous and exogenous stimuli, including infection by bacteria, cells C8-4B were treated with 100 ng/µl lipopolysaccharide viruses, prions and β-amyloid plaques (1). Microglia are (LPS) and 1 ng/µl interferon-γ. Cells were subsequently treated activated upon exposure to different stimuli and, depending with 2% isoflurane, 100 µM callistephin or both. LPS promoted on the environmental context, this may be beneficial or detri- apoptosis in C8-B4 cells, and this was reduced following mental to the functionality and physiology of the CNS (2). -

Anthocyanins: Antioxidant And/Or Anti-Inflammatory Activities
Journal of Applied Pharmaceutical Science 01 (06); 2011: 07-15 ISSN: 2231-3354 Anthocyanins: Antioxidant and/or anti-inflammatory Received on: 18-08-2011 Accepted on: 23-08-2011 activities M. G. Miguel ABSTRACT Anthocyanins are polyphenols with known antioxidant activity which may be responsible for some biological activities including the prevention or lowering the risk of cardiovascular disease, diabetes, arthritis and cancer. Nevertheless such properties, their stability and bioavailability depend M. G. Miguel on their chemical structure. In the present work a brief review is made on chemical structures, Faculdade de Ciências e Tecnologia, bioavailability and antioxidant/anti -inflammatory of anthocyanins. Departamento de Química e Farmácia, Centro de Biotecnologia Vegetal, Instituto de Biotecnologia e Key words: Anthocyanins, chemistry, stability, bioavailability, free radical scavenging. Bioengenharia, Universidade do Algarve, Campus de Gambelas, 8005- 139 Faro, PORTUGAL INTRODUCTION Anthocyanins are generally accepted as the largest and most important group of water- soluble pigments in nature (Harborne, 1998). They are responsible for the blue, purple, red and orange colors of many fruits and vegetables. The word anthocyanin derived from two Greek words: anthos, which means flowers, and kyanos, which means dark blue (Horbowicz et al., 2008). Major sources of anthocyanins are blueberries, cherries, raspberries, strawberries, black currants, purple grapes and red wine (Mazza, 2007). They belong to the family of compounds known as flavonoids, but they are distinguished from other flavonoids due to their capacity to form flavylium cations (Fig. 1) (Mazza, 2007). + O Fig 1. Flavylium cation. They occur principally as glycosides of their respective aglycone anthocyanidin- chromophores with the sugar moiety generally attached at the 3-position on the C-ring or the 5- position on the A-ring (Prior and Wu, 2006). -

Phenolics in Human Health
International Journal of Chemical Engineering and Applications, Vol. 5, No. 5, October 2014 Phenolics in Human Health T. Ozcan, A. Akpinar-Bayizit, L. Yilmaz-Ersan, and B. Delikanli with proteins. The high antioxidant capacity makes Abstract—Recent research focuses on health benefits of polyphenols as an important key factor which is involved in phytochemicals, especially antioxidant and antimicrobial the chemical defense of plants against pathogens and properties of phenolic compounds, which is known to exert predators and in plant-plant interferences [9]. preventive activity against infectious and degenerative diseases, inflammation and allergies via antioxidant, antimicrobial and proteins/enzymes neutralization/modulation mechanisms. Phenolic compounds are reactive metabolites in a wide range of plant-derived foods and mainly divided in four groups: phenolic acids, flavonoids, stilbenes and tannins. They work as terminators of free radicals and chelators of metal ions that are capable of catalyzing lipid oxidation. Therefore, this review examines the functional properties of phenolics. Index Terms—Health, functional, phenolic compounds. I. INTRODUCTION In recent years, fruits and vegetables receive considerable interest depending on type, number, and mode of action of the different components, so called as “phytochemicals”, for their presumed role in the prevention of various chronic diseases including cancers and cardiovascular diseases. Plants are rich sources of functional dietary micronutrients, fibers and phytochemicals, such -

Phytochemistry and Pharmacological Activities of Clitoria Ternatea
International Journal of Natural and Social Sciences, 2017, 4(1): 01-10 ISSN: 2313-4461 Phytochemistry and pharmacological activities of Clitoria ternatea Md. Bakhtiar Lijon1*, Nigar Sultana Meghla2, Eleas Jahedi1, Md. Abdur Rahman1, Ismail Hossain1 1Modern Food Testing Laboratory, Chittagong City Corporation, Chittagong, Bangladesh 2Department of Microbiology, Jessore University of Science and Technology, Bangladesh ARTICLE INFO ABSTRACT Article history Clitoria ternatea is a twining herbal medicinal plant mostly found in Asia. Various constituents are found in different parts of the plant. The plant Clitotia ternatea is traditionally used for Accepted 03 Jan 2017 food coloring, stress, infertility and gonorrhea. The plant has been widely used in Ayurveda. Online release 09 Jan 2017 Pharmacologically it is an anxiolytic, anti inflammatory, analgesic, anti-microbial and anti carcinogenic. It is also Cns Depressant, nephroprotective and has anti-Stress activities. Keyword Generally Clitoria ternatea has larvicidal activities, proteolytic activities, antihelmintic activities, antihyperglycenmic activity, diuretic activity, antioxidant activity, antihistaminic Clitoria ternatea activity and treat goiter. As Clitoria ternatea plant has great usefulness it should be cultivated, Morphology conserve and further research should be conducted for human well being. This article will Phytochemistry focus on pharmaco-chemical characterization of Clitoria ternatea with the traditional and Pharmacological activity pharmacological uses of Clitoria ternatea. *Corresponding Author Md. Bakhtiar Lijon [email protected] INTRODUCTION function (Ramaswamy et al., 2011). It is also used in the treatment of chronic bronchitis, dropsy, Plants and herbs have been an important goiter, leprosy, mucous disorders, sight weakness, contributor to the quality of human life for skin diseases, sore throat and tumors (Ramaswamy thousands of years. -

Characterisation of Bioactive Compounds in Berries from Plants Grown Under Innovative Photovoltaic Greenhouses
Journal of Berry Research 8 (2018) 55–69 55 DOI:10.3233/JBR-170258 IOS Press Characterisation of bioactive compounds in berries from plants grown under innovative photovoltaic greenhouses Federica Blandoa,∗, Carmela Gerardia, Massimiliano Rennab,c, Sergio Castellanod and Francesco Seriob aInstitute of Sciences of Food Production (ISPA), CNR, Lecce, Italy bInstitute of Sciences of Food Production (ISPA), CNR, Bari, Italy cDepartment of Agricultural and Environmental Science, University of Bari Aldo Moro, Bari, Italy dDepartment of Science of Agriculture, Food and Environment, (SAFE) University of Foggia, Foggia, Italy Received 2 July 2017; accepted 1 November 2017 Abstract. BACKGROUND: Bioactive compounds, mainly polyphenols, present in berries, are thought to be responsible for the health benefits of these fruit. Therefore, it is worthwhile to define the optimal environmental conditions to maximise their polyphenol content. OBJECTIVE: With the aim to define the optimal conditions for berry cultivation in an innovative environment, red rasp- berry, wild strawberry and blackberry plants were grown in a traditional greenhouse in comparison with two photovoltaic greenhouses with different shading area. METHODS: Hydroalcoholic extracts of ripe berries were evaluated by HPLC analysis, for their anthocyanins, organic acids and sugar contents. Moreover, phenolic content (by the Folin-Ciocalteu assay) and antioxidant activity (by the Trolox equivalent antioxidant capacity-TEAC assay) were assayed on the same berry extracts. RESULTS: Total anthocyanins,