Advanced Selection Materials in Solar Cell Efficiency and Their Properties- a Comprehensive Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Life Cycle Analysis of High-Performance Monocrystalline Silicon Photovoltaic Systems: Energy Payback Times and Net Energy Production Value
27th European Photovoltaic Solar Energy Conference and Exhibition LIFE CYCLE ANALYSIS OF HIGH-PERFORMANCE MONOCRYSTALLINE SILICON PHOTOVOLTAIC SYSTEMS: ENERGY PAYBACK TIMES AND NET ENERGY PRODUCTION VALUE Vasilis Fthenakis1,2, Rick Betita2, Mark Shields3, Rob Vinje3, Julie Blunden3 1 Brookhaven National Laboratory, Upton, NY, USA, tel. 631-344-2830, fax. 631-344-3957, [email protected] 2Center for Life Cycle Analysis, Columbia University, New York, NY 10027, USA 3SunPower Corporation, San Jose, CA, USA ABSTRACT: This paper summarizes a comprehensive life cycle analysis based on actual process data from the manufacturing of Sunpower 20.1% efficient modules in the Philippines and other countries. Higher efficiencies are produced by innovative cell designs and material and energy inventories that are different from those in the production of average crystalline silicon panels. On the other hand, higher efficiencies result to lower system environmental footprints as the system area on a kW basis is smaller. It was found that high efficiencies result to a net gain in environmental metrics (i.e., Energy Payback Times, GHG emissions) in comparison to average efficiency c-Si modules. The EPBT for the Sunpower modules produced in the Philippines and installed in average US or South European insolation is only 1.4 years, whereas the lowest EPBT from average efficiency c-Si systems is ~1.7 yrs. To capture the advantage of high performance systems beyond their Energy Payback Times, we introduced the metric of Net Energy Production Value (NEPV), which shows the solar electricity production after the system has “paid-off” the energy used in its life-cycle. The SunPower modules are shown to produce 45% more electricity than average efficiency (i.e., 14%) c-Si PV modules. -

New Challenges and Possibilities for Silicon Based Solar Cells
1 New challenges and possibilities for silicon based solar cells Marisa Di Sabatino Dept of Materials Science and Engineering NTNU 2 Outline -What is a solar cell? -How does it work? -Silicon based solar cells manufacturing -Challenges and Possibilities -Concluding remarks 3 What is a solar cell? • It is a device that converts the energy of the sunlight directly into electricity. CB PHOTON Eph=hc/λ VB Electron-hole pair 4 How does a solar cell work? 5 How does a solar cell work? Current Semiconductor Eg + - P-n junction Metallic contacts Turid Worren Reenaas 6 How does a solar cell work? - Charge generation (electron-hole pairs) - Charge separation (electric field) - Charge transport 7 - Charge generation (electron-hole pairs) - Charge separation (electric field) - Charge transport Eph>>Eg Eph<<Eg Eph>Eg Eg CB Eph VB 8 - Charge generation (electron-hole pairs) - Charge separation (electric field) - Charge transport p-type C - - - - Fermi level - + + + + + n-type n-p junction v + - Electric field 9 - Charge generation (electron-hole pairs) - Charge separation (electric field) - Charge transport Back and front side of a silicon solar cell 10 Semiconductors Materials for solar cells 11 Why Silicon? Silicon: abundant, cheap, well-known technology 12 Silicon solar cell value chain 13 Silicon solar cells value chain From sand to solar cells… Raw Materials Efficiency Crystallization Isc, Voc, FF Feedstock Solar cell process Photo: Melinda Gaal 14 Multicrystalline silicon solar cells Directional solidification 15 Monocrystalline silicon solar cells Czochralski process 16 Crystallization methods for PV silicon • Multicrystalline silicon ingots: – Lower cost than monocrystalline – More defects (dislocations and impurities) • Monocrystalline silicon ingots: – Higher cost and lower yield – Oxygen related defects – Structure loss 17 Czochralski PV single crystal growth • Dominating process for single crystals • Both p (B-doped) and n (P-doped) type crystals • Growth rate 60 mm/h Challenges: •Productivity low due to slow growth, long cycle time.. -

15Th Workshop on Crystalline Silicon Solar Cells and Modules: Materials and Processes
A national laboratory of the U.S. Department of Energy Office of Energy Efficiency & Renewable Energy National Renewable Energy Laboratory Innovation for Our Energy Future th Proceedings 15 Workshop on Crystalline NREL/BK-520-38573 Silicon Solar Cells and Modules: November 2005 Materials and Processes Extended Abstracts and Papers Workshop Chairman/Editor: B.L. Sopori Program Committee: M. Al-Jassim, J. Kalejs, J. Rand, T. Saitoh, R. Sinton, M. Stavola, R. Swanson, T. Tan, E. Weber, J. Werner, and B. Sopori Vail Cascade Resort Vail, Colorado August 7–10, 2005 NREL is operated by Midwest Research Institute ● Battelle Contract No. DE-AC36-99-GO10337 th Proceedings 15 Workshop on Crystalline NREL/BK-520-38573 Silicon Solar Cells and Modules: November 2005 Materials and Processes Extended Abstracts and Papers Workshop Chairman/Editor: B.L. Sopori Program Committee: M. Al-Jassim, J. Kalejs, J. Rand, T. Saitoh, R. Sinton, M. Stavola, R. Swanson, T. Tan, E. Weber, J. Werner, and B. Sopori Vail Cascade Resort Vail, Colorado August 7–10, 2005 Prepared under Task No. WO97G400 National Renewable Energy Laboratory 1617 Cole Boulevard, Golden, Colorado 80401-3393 303-275-3000 • www.nrel.gov Operated for the U.S. Department of Energy Office of Energy Efficiency and Renewable Energy by Midwest Research Institute • Battelle Contract No. DE-AC36-99-GO10337 NOTICE This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or `process disclosed, or represents that its use would not infringe privately owned rights. -

A Review Paper Ondevelopment in Material Used in Solar Pannel As
SSRG International Journal of Mechanical Engineering Volume 6 Issue 6, 35-41, June 2019 ISSN: 2348–8360 /doi: 10.14445/23488360/IJME-V6I6P107 © 2019 Seventh Sense Research Group® A Review Paper on Development in Material Used in Solar Pannel as Solar Cell Material Ankur Kumar Bansal, Dinesh Kumar, Dr. Mukesh Kumar M.tech Mechanical, AKTU Lucknow, India Abstract electron flow when photons from sunlight absorbed In the present era, While seeing the increasing and ejected electrons, leaving a hole that is further demand for energy and depletion of resources from filled by the surrounding electrons. This phenomenon which we obtained energy, solar energy suggest as is called the photovoltaic effect. The PV cell directs the best alternative energy resource. The light from the electrons in one direction, which gives rise to the the sun is not only a non-vanishing resource of flow of current. The amount of current is directly energy but also it is an Eco- Friendly resource of proportional to the humble of absorbed photons. So it energy(free from the environment pollution and can be easily said that PV cells are a variable current noise). It can easily compensate for the energy source. The first solar cell was built by Charles Fritts requirements fulfilled by the other resources, which in 1883 by the use of a thin layer of gold, and a are depleting and environment challenges, such as coating of selenium formed a junction. In the starting Fossil Fuel and petroleum deposits. era, the PCE was very low, about 1% only, but Basically, we receive solar energy from the further improvements make it increase. -

Research Into Fabrication and Popularization of Organic Thin Film Solar Cells, Chemical Engineering Transactions, 55, 25-30 DOI:10.3303/CET1655005 26
25 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 55, 2016 The Italian Association of Chemical Engineering Online at www.aidic.it/cet Guest Editors: Tichun Wang, Hongyang Zhang, Lei Tian Copyright © 2016, AIDIC Servizi S.r.l., ISBN 978-88-95608-46-4; ISSN 2283-9216 DOI: 10.3303/CET1655005 Research into Fabrication and Popularization of Organic Thin Film Solar Cells Bin Zhang*a, Yan Lia, Shanlin Qiaob, Le Lic, Zhanwen Wanga a Hebei Chemical & Pharmaceutical College, No. 88 Fangxing Road, Shijiazhuang, Hebei Province, China; b Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, No. 189 Songling Road, Qingdao, Shandong Province, China c Shijiazhuang Naienph Chemical Technology Co., Ltd, No. 12 Shifang Road, Shijiazhuang, Hebei Province, China. [email protected] An analysis was conducted herein on the research status of several popular solar cells at the present stage, including silicon solar cell, thin film photovoltaic cell, and dye-sensitized solar cell (DSSC). In doing so, we concluded that the current situations provide a favorable objective environment for the popularization of organic thin film solar cells. Finally, we reviewed the merits and demerits of the organic thin film solar cell together with the major research focus on and progress of it, and summarized obstacles to and development trails of the popularization of organic thin film solar cells. 1. Introduction As the energy crisis further deepens in the 21st century, the existing development level for solar cells has already failed to satisfy increasing social demands for energy. This phenomenon is mainly reflected in the costly high-purity silicon solar panels, in the defects at new amorphous silicon (a-Si) during energy conversion, and in the limited theoretical energy conversion efficiency (around 25%) of silicon solar panels as well. -
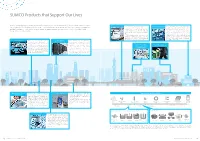
SUMCO Products That Support Our Lives
SUMCO Products that Support Our Lives SUMCO manufactures silicon wafers, a key material in semiconductor devices. Semiconductor devices that use SUMCO’s silicon Trains, Bullet Trains Devices called power semiconductors are Medical Equipments In the medical field, medical equipment has wafers support our lives in a variety of ways, from electronic devices around us such as mobile phones, computers, smartphones used to control electric power. These devices continued to evolve, with the advent of high- and digital appliances to automobiles, medical equipment, industrial machinery control units, as well as the control of public are technically complex and require reliable precision diagnostic imaging equipment and transportation and infrastructure. control of large amounts of power and power surgery robots capable of precise control. saving performance, making this a specialized A large number of silicon wafers are used field. in these medical devices, and the silicon Power supply control for heavy electric wafers that serve as their substrates require machinery, in particular, such as electric trains high reliability, especially as human lives are that use power of over 1000V, requires special involved. SUMCO’s silicon wafers contribute to know-how for the silicon wafers, as well. the advance of medicine. Automobiles Numerous semiconductor devices Data Centers With smartphones and computers are at work inside motor vehicles. An (Server Rooms) becoming increasingly sophisticated, extremely high level of quality and vast quantities of data in the form of Power Generation reliability is required for silicon wafers high-quality photographs and vid- Smart Card Devices Facilities and Public to be used for motor control in electric eos are being processed in the cloud vehicles (EV) and hybrid vehicles (HV/ and stored in data centers. -

Solar Cells They Rely on Are Notoriously Expensive to Produce
THE PRESENT PROBLEM WITH SOLAR POWER is price. Ironically, sunlight, which is abundant beyond the energy needs of the entire human race and completely free, is frequently deemed too expensive to harness. Photovoltaic panels, systems to make them compatible with grid electricity, and batteries to squirrel away energy for when it’s cloudy—all these add cost. And while such hardware and installation costs will continue to diminish over time, the standard silicon solar cells they rely on are notoriously expensive to produce. Naturally, the scientific community has taken great interest in identifying alternative materials for solar cells. Solar-harvesting materials under development at Los Alamos and elsewhere include specialized thin films, organic layers, semi- conductor nanodevices, and others. Each has promise, and each has drawbacks. But a new class of challengers emerged a few years ago and has been improving with surprising speed since then. Known as perovskites, they are any crystalline material with the same broad class of chemical structure as a natural mineral of the same name. Perovskite solar cells are generally easy to work with, easy to adjust for improved performance, and very easy to afford. And in recent experi- mentation at Los Alamos, a particular recipe has been shown to reliably generate perovskite crystals that exhibit solar conversion efficiencies comparable to those of silicon. “Silicon solar cells are still the gold standard. They’re reliable and efficient, and they’ve been thoroughly demonstrated in the field,” says Los Alamos materials scientist Aditya Mohite. “I can’t wait to render them obsolete.” Anatomy of a cell A standard solar cell contains an active layer, usually silicon, sandwiched between two electrode layers. -

Emerging Next Generation Solar Cells: Route to High Efficiency and Low Cost
International Journal of Trend in Scientific Research and Development, Volume 1(4), ISSN: 2456-6470 www.ijtsrd.com Emerging Next Generation Solar Cells: Route to High Efficiency and Low Cost M. S. Sadek* M. J. Rashid Dept. of Electrical and Electronic Engineering, Dept. of Electrical and Electronic Engineering, Southeast University, University of Dhaka, Dhaka, Bangladesh Dhaka, Bangladesh M.A. Zaman Z. H. Mahmood Dept. of Electrical and Electronic Engineering, Prime Dept. of Electrical and Electronic Engineering, University University of Dhaka, Dhaka, Bangladesh Dhaka, Bangladesh Abstract including their structural morphology, electrical and Generation of clean energy is one of the main optical properties. The excellent state of the art challenges of the 21st century. Solar energy is the technology, advantages and potential research issues most abundantly available renewable energy source yet to be explored are also pointed out. which would be supplying more than 50% of the global electricity demand in 2100.Solar cells are used Keywords -Solar Cells, Thin film, Silicon, Organic, to convert light energy into electrical energy directly Perovskite, DSSC, CZTS, Efficiency, Costs. with an appeal that it does not generate any harmful bi-products, like greenhouse gasses. The I. INTRODUCTION manufacturing of solar cells is actually based on the types of semiconducting or non-semiconducting Based on different technologies and materials, the materials used and commercial maturity. From the solar cells can be grouped into three different very beginning of the terrestrial use of Solar Cells, generations. First generation or c-Si solar cells are efficiency and costs are the main focusing areas of most dominant photovoltaic (PV) technology even research. -

Solar Frontier Breaks CZTS Cell Efficiency Record World-Record 12.6% Cell Efficiency Achieved with IBM and TOK
NEWS RELEASE Solar Frontier Breaks CZTS Cell Efficiency Record World-record 12.6% cell efficiency achieved with IBM and TOK Tokyo-December 10, 2012 -Solar Frontier announced today that it has set a new conversion efficiency record for CZTS solar cells (0.42cm²) at 12.6%. The latest world record was achieved in joint research with IBM (NYSE:IBM) and Tokyo Ohka Kogyo (TOK), and has been independently verified by Newport Corporation1. The details have been published in the Advanced Energy Material, journal on Nov 272. CZTS stands for key ingredients copper, zinc, tin, sulphur and selenium. Inexpensive and with abundant supply, these materials offer CZTS a cost-competitive advantage over other photovoltaic technologies. The new 12.6% world record eclipses the previous record of 11.1%, which was also set by Solar Frontier and its joint-research partners. “Breaking our previous record at such a fast pace shows the potential of CZTS for mass production in the future, and we are now in a position to drive that efficiency even higher,” said Satoru Kuriyagawa, Chief Technology Officer of Solar Frontier. Solar Frontier is the world’s largest provider of CIS thin-film photovoltaic solutions. Its Atsugi Research Center in Kanagawa, Japan, has also achieved standing world record conversion efficiencies of 17.8% on a 30cm by 30cm CIS submodule and 19.7% for non-cadmium CIS cell. Solar Frontier’s CIS modules, manufactured in Japan, are offered in commercial volumes at up to 13.8% efficiency, the highest of any mass produced thin- film modules. Picture provided by IBM 1 Newport Corporation provides advanced technology products and solutions, including photovoltaic conversion efficiency certification. -

Summary of the High-Efficiency Crystalline Solar Cell Research Forum
SUMMARY OF THE HIGH-EFFICIENCY CRYSTALLINE SOLAR CELL RESEARCH FORUM UNIVERSITY OF PENNSYLVANIA M. Wolf Session I: OVERVIEW P. Landsberg Some Aspects of the Minority Carrier Lifetime in Silicon. C.T. Sah Review of Recombination Phenomena in Righ- Efficiency Solar Cells. Session 11: EGR EFFICIENCY CONCEPTS n. Wolf Silicon Solar Cell Efficiency Improvement: Status and Outlook. Some Practical Considerattons far Econoaical Back Contact Fovaaaticn on High-Efficiency Solar Cells. High-Efficiency Cell Concepts on Low-Cost Silicon Sheet. R, Swanson High Lifetime Silicon Processing. L. Olsen Silicon HINP Solar Cells. Session 111: SURFACE/INTERFACE EFFECTS D. Chadi Atomic Structure of the Annealed Si (111) Surface. L. Kazaerski Surface and Interface Characteristics, S. Lai Nitridation of SiO2 for Surface Passivatfon. S. Panash Surface Passivation and Junction Formation Using Low-Energy Hydrogen Implants. P, Grunthaner Chemical Structure of Interfaces. PLENARY SESS!ONS Session IV: BULK BPFRCTS E. Sirtl Structural Defects in Crystalline Silicon. C. Pierce Oxygen and Carbon Impurities and Related Defects in Silicon. T. Tan Current Understanding of Point Defects and Diffusion Processes in Silicon. G. Schwuttke Defects in Web Dendrite Silicon Ribbon Crystals and Their Influences on Hinority Carrier Lifetime. J. Hanoka EBLC Characterization and dydrogen Passivation in Silicon Sheet. A. Neugrsschel Measurement of Electrical Parameters and Current Components in the Bulk of Silicon Solar Cells. Session V: MODEL-- I NG - R. Schwartz Current Status of One and Two Dimensional Numer- ical Models: Successes and Limitations. M. Lamorte Application of Closed-Form Solution Using Re- cursion Relationship in Silicon Solar Cells. P. Lindholm PIenomena Simulation for Heavy Doping and Surface ! Recombination Velocity. -

A New Slicing Method of Monocrystalline Silicon Ingot by Wire EDM
cf~ Paper International Journal of Electrical Machining, No. 8, January 2003 A New Slicing Method of Monocrystalline Silicon Ingot by Wire EDM Akira OKADA *, Yoshiyuki UNO *, Yasuhiro OKAMOTO*, Hisashi ITOH* and Tameyoshi HlRANO** (Received May 28, 2002) * Department of Mechanical Engineering, Okayama University, Okayama 700-8530, Japan ** Toyo Advanced Technologies Co., Ltd., Hiroshima 734-8501, Japan Abstract Monocrystalline silicon is one of the most important materials in the semiconductor industry because of its many excellent properties as a semiconductor. In the manufacturing process of silicon wafers, inner diameter(lD) blade and multi wire saw have conventionally been used for slicing silicon ingots. However, some problems in efficiency, accuracy, slurry treatment and contamination are experienced when applying this method to large scale wafers of 12 or 16 inch diameter expected to be used in the near future. Thus, the improvement of conventional methods or a new slicing method is strongly required. In this study, the possibility of slicing a silicon ingot by wire EDM was discussed and the machining properties were experimentally investigated. A silicon wafer used as substrate for epitaxial film growth has low resistivity in the order of 0.01 g .cm, which makes it possible to cut silicon ingots by wire EDM. It was clarified that the new wire EDM has potential for application as a new slicing method, and that the surface roughness using this method is as small as that using the conventional multi wire saw method. Moreover, it was pointed out that the contamination due to the adhesion and diffusion of wire electrode material into the machined surface can be reduced by wire EDM under the condition of low current and long discharge duration. -

A Study on the Countermeasures and Suggestions of Technology
4th International Education, Economics, Social Science, Arts, Sports and Management Engineering Conference (IEESASM 2016) A Study on the Countermeasures and Suggestions of Technology Transfer in the Solar Cells Industry in China Lvcheng Lia, Guoping Chengb School of Management, Wuhan University of Technology, Wuhan 430070, China [email protected], [email protected] Keywords: solar cells; technology transfer; countermeasures and suggestions Abstract. This paper analyzes the development and industrialization of solar cells of three generations: mono-crystalline silicon and polycrystalline silicon solar cell, thin-film solar cell and nanocrystalline solar cell. It is believed that technology transfer and university-industry collaboration play important roles in the solar cells industry; and put forward reasonable suggestions for improvements from the aspects of financial subsidies, intellectual property, risk compensation system, technology transfer intermediary and cultivation of talents. 1. Introduction New energy technology is one of the most important topics for human's development in the 21st century. The traditional energy sources such as oil and natural gas, have some serious problems like pollution and storage, which cannot satisfy the requirements of human beings and conflict with the sustainable development. As an important component of new energy, solar energy exhibits the advantages of low carbon emission, clean production, flexibility and renewability, which is ideal to be applied as a new alternative energy and has become the focus of the scientific community and industry. Presently, the solar cells have already been used in national defense, communications, computers, transportation and many other fields. Due to China's unique geographical advantages, to develop the solar energy is the trend of the time.