6.701 Introduction to Nanoelectronics, Part 2: the Quantum Particle in A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
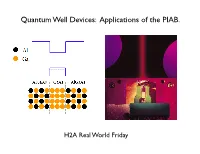
Quantum Well Devices: Applications of the PIAB
Quantum Well Devices: Applications of the PIAB. H2A Real World Friday What is a semiconductor? no e- Conduction Band Empty e levels a few e- Empty e levels lots of e- Empty e levels Filled e levels Filled e levels Filled e levels Insulator Semiconductor Metal Electrons in the conduction band of semiconductors like Si or GaAs can move about freely. Conduction band a few e- Eg = hν Energy Bandgap in Valence band Filled e levels Semiconductors We can get electrons into the conduction band by either thermal excitation or light excitation (photons). Solar cells use semiconductors to convert photons to electrons. A "quantum well" structure made from AlGaAs-GaAs-AlGaAs creates a potential well for conduction electrons. 10-20 nm! A conduction electron that get trapped in a quantum well acts like a PIAB. A conduction electron that get trapped in a quantum well acts like a PIAB. Quantum Wells are used to make Laser Diodes Quantum Well Laser Diodes Quantum Wells are used to make Laser Diodes Quantum Well Laser Diodes Multiple Quantum Wells work even better. Multiple Quantum Well Laser Diodes Multiple Quantum Wells work even better. Multiple Quantum Well LEDs Multiple Quantum Wells work even better. Multiple Quantum Well Laser Diodes Multiple Quantum Wells also are used to make high efficiency Solar Cells. Quantum Well Solar Cells Multiple Quantum Wells also are used to make high efficiency Solar Cells. The most common approach to high efficiency photovoltaic power conversion is to partition the solar spectrum into separate bands and each absorbed by a cell specially tailored for that spectral band. -

Quantum Dots
Quantum Dots www.nano4me.org © 2018 The Pennsylvania State University Quantum Dots 1 Outline • Introduction • Quantum Confinement • QD Synthesis – Colloidal Methods – Epitaxial Growth • Applications – Biological – Light Emitters – Additional Applications www.nano4me.org © 2018 The Pennsylvania State University Quantum Dots 2 Introduction Definition: • Quantum dots (QD) are nanoparticles/structures that exhibit 3 dimensional quantum confinement, which leads to many unique optical and transport properties. Lin-Wang Wang, National Energy Research Scientific Computing Center at Lawrence Berkeley National Laboratory. <http://www.nersc.gov> GaAs Quantum dot containing just 465 atoms. www.nano4me.org © 2018 The Pennsylvania State University Quantum Dots 3 Introduction • Quantum dots are usually regarded as semiconductors by definition. • Similar behavior is observed in some metals. Therefore, in some cases it may be acceptable to speak about metal quantum dots. • Typically, quantum dots are composed of groups II-VI, III-V, and IV-VI materials. • QDs are bandgap tunable by size which means their optical and electrical properties can be engineered to meet specific applications. www.nano4me.org © 2018 The Pennsylvania State University Quantum Dots 4 Quantum Confinement Definition: • Quantum Confinement is the spatial confinement of electron-hole pairs (excitons) in one or more dimensions within a material. – 1D confinement: Quantum Wells – 2D confinement: Quantum Wire – 3D confinement: Quantum Dot • Quantum confinement is more prominent in semiconductors because they have an energy gap in their electronic band structure. • Metals do not have a bandgap, so quantum size effects are less prevalent. Quantum confinement is only observed at dimensions below 2 nm. www.nano4me.org © 2018 The Pennsylvania State University Quantum Dots 5 Quantum Confinement • Recall that when atoms are brought together in a bulk material the number of energy states increases substantially to form nearly continuous bands of states. -

Interaction Effects in a Microscopic Quantum Wire Model with Strong OPEN ACCESS Spin–Orbit Interaction
PAPER • OPEN ACCESS Related content - Correlation effects in two-dimensional Interaction effects in a microscopic quantum wire topological insulators M Hohenadler and F F Assaad model with strong spin–orbit interaction - Charge dynamics of the antiferromagnetically ordered Mott insulator To cite this article: G W Winkler et al 2017 New J. Phys. 19 063009 Xing-Jie Han, Yu Liu, Zhi-Yuan Liu et al. - One-dimensional Fermi liquids J Voit View the article online for updates and enhancements. This content was downloaded from IP address 131.211.105.193 on 19/02/2018 at 15:46 New J. Phys. 19 (2017) 063009 https://doi.org/10.1088/1367-2630/aa7027 PAPER Interaction effects in a microscopic quantum wire model with strong OPEN ACCESS spin–orbit interaction RECEIVED 16 January 2017 G W Winkler1,2, M Ganahl2,3, D Schuricht4, H G Evertz2,5 and S Andergassen6,7 REVISED 1 Theoretical Physics and Station Q Zurich, ETH Zurich, 8093 Zurich, Switzerland 9 April 2017 2 Institute of Theoretical and Computational Physics, Graz University of Technology, A-8010 Graz, Austria ACCEPTED FOR PUBLICATION 3 Perimeter Institute for Theoretical Physics, Waterloo, Ontario N2L 2Y5, Canada 28 April 2017 4 Institute for Theoretical Physics, Center for Extreme Matter and Emergent Phenomena, Utrecht University, Princetonplein 5, 3584 CE PUBLISHED Utrecht, The Netherlands 5 June 2017 5 Kavli Institute for Theoretical Physics, University of California, Santa Barbara, CA 93106, United States of America 6 Institute for Theoretical Physics and Center for Quantum Science, Universität Tübingen, Auf der Morgenstelle 14, D-72076 Tübingen, Original content from this Germany work may be used under 7 Faculty of Physics, University of Vienna, Boltzmanngasse 5, A-1090 Vienna, Austria the terms of the Creative Commons Attribution 3.0 E-mail: [email protected] licence. -

Quantized Transmission in an Asymmetrically Biased Quantum Point Contact
Linköping University | Department of Physics, Chemistry and Biology Master’s thesis, 30 hp | Master’s programme in Physics and Nanoscience Autumn term 2016 | LITH-IFM-A-EX—16/3274--SE Quantized Transmission in an Asymmetrically Biased Quantum Point Contact Erik Johansson Examinator, Magnus Johansson Supervisors, Irina Yakimenko & Karl-Fredrik Berggren Avdelning, institution Datum Division, Department Date Theoretical Physics 2016-11-07 Department of Physics, Chemistry and Biology Linköping University, SE-581 83 Linköping, Sweden Språk Rapporttyp ISBN Language Report category Svenska/Swedish Licentiatavhandling ISRN: LITH-IFM-A-EX--16/3274--SE Engelska/English Examensarbete _________________________________________________________________ C-uppsats D-uppsats Serietitel och serienummer ISSN ________________ Övrig rapport Title of series, numbering ______________________________ _____________ URL för elektronisk version Titel Title Quantized Transmission in an Asymmetrically Biased Quantum Point Contact Författare Author Erik Johansson Sammanfattning Abstract In this project work we have studied how a two-dimensional electron gas (2DEG) in a GaAs/AlGaAs semiconductor heterostructure can be locally confined down to a narrow bottleneck constriction called a quantum point contact (QPC) and form an artificial quantum wire using a split-gate technique by application of negative bias voltages. The electron transport through the QPC and how asymmetric loading of bias voltages affects the nature of quantized conductance were studied. The basis is -

Dephasing in an Electronic Mach-Zehnder Interferometer,” Phys
Dephasing and Quantum Noise in an electronic Mach-Zehnder Interferometer Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften (Dr. rer. nat.) der Fakultät für Physik der Universität Regensburg vorgelegt von Andreas Helzel aus Kelheim Dezember 2012 ii Promotionsgesuch eingereicht am: 22.11.2012 Die Arbeit wurde angeleitet von: Prof. Dr. Christoph Strunk Prüfungsausschuss: Prof. Dr. G. Bali (Vorsitzender) Prof. Dr. Ch. Strunk (1. Gutachter) Dr. F. Pierre (2. Gutachter) Prof. Dr. F. Gießibl (weiterer Prüfer) iii In Gedenken an Maria Höpfl. Sie war die erste Taxifahrerin von Kelheim und wurde 100 Jahre alt. Contents 1. Introduction1 2. Basics 5 2.1. The two dimensional electron gas....................5 2.2. The quantum Hall effect - Quantized Landau levels...........8 2.3. Transport in the quantum Hall regime.................. 10 2.3.1. Quantum Hall edge states and Landauer-Büttiker formalism.. 10 2.3.2. Compressible and incompressible strips............. 14 2.3.3. Luttinger liquid in the QH regime at filling factor 2....... 16 2.4. Non-equilibrium fluctuations of a QPC.................. 24 2.5. Aharonov-Bohm Interferometry..................... 28 2.6. The electronic Mach-Zehnder interferometer............... 30 3. Measurement techniques 37 3.1. Cryostat and devices........................... 37 3.2. Measurement approach.......................... 39 4. Sample fabrication and characterization 43 4.1. Fabrication................................ 43 4.1.1. Material.............................. 43 4.1.2. Lithography............................ 44 4.1.3. Gold air bridges.......................... 45 4.1.4. Sample Design.......................... 46 4.2. Characterization.............................. 47 4.2.1. Filling factor........................... 47 4.2.2. Quantum point contacts..................... 49 4.2.3. Gate setting............................ 50 5. Characteristics of a MZI 51 5.1. -

Hyperbolic Metamaterials Based on Quantum-Dot Plasmon-Resonator Nanocomposites
Downloaded from orbit.dtu.dk on: Oct 04, 2021 Hyperbolic metamaterials based on quantum-dot plasmon-resonator nanocomposites. Zhukovsky, Sergei; Ozel, T.; Mutlugun, E.; Gaponik, N.; Eychmuller, A.; Lavrinenko, Andrei; Demir, H. V.; Gaponenko, S. V. Published in: Optics Express Link to article, DOI: 10.1364/OE.22.018290 Publication date: 2014 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Zhukovsky, S., Ozel, T., Mutlugun, E., Gaponik, N., Eychmuller, A., Lavrinenko, A., Demir, H. V., & Gaponenko, S. V. (2014). Hyperbolic metamaterials based on quantum-dot plasmon-resonator nanocomposites. Optics Express, 22(15), 18290-18298. https://doi.org/10.1364/OE.22.018290 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Hyperbolic metamaterials based on quantum-dot plasmon-resonator nanocomposites 1, 2 2,3 4 S. V. Zhukovsky, ∗ T. Ozel, E. Mutlugun, N. Gaponik, A. -

Quantum Dot and Electron Acceptor Nano-Heterojunction For
www.nature.com/scientificreports OPEN Quantum dot and electron acceptor nano‑heterojunction for photo‑induced capacitive charge‑transfer Onuralp Karatum1, Guncem Ozgun Eren2, Rustamzhon Melikov1, Asim Onal3, Cleva W. Ow‑Yang4,5, Mehmet Sahin6 & Sedat Nizamoglu1,2,3* Capacitive charge transfer at the electrode/electrolyte interface is a biocompatible mechanism for the stimulation of neurons. Although quantum dots showed their potential for photostimulation device architectures, dominant photoelectrochemical charge transfer combined with heavy‑metal content in such architectures hinders their safe use. In this study, we demonstrate heavy‑metal‑free quantum dot‑based nano‑heterojunction devices that generate capacitive photoresponse. For that, we formed a novel form of nano‑heterojunctions using type‑II InP/ZnO/ZnS core/shell/shell quantum dot as the donor and a fullerene derivative of PCBM as the electron acceptor. The reduced electron–hole wavefunction overlap of 0.52 due to type‑II band alignment of the quantum dot and the passivation of the trap states indicated by the high photoluminescence quantum yield of 70% led to the domination of photoinduced capacitive charge transfer at an optimum donor–acceptor ratio. This study paves the way toward safe and efcient nanoengineered quantum dot‑based next‑generation photostimulation devices. Neural interfaces that can supply electrical current to the cells and tissues play a central role in the understanding of the nervous system. Proper design and engineering of such biointerfaces enables the extracellular modulation of the neural activity, which leads to possible treatments of neurological diseases like retinal degeneration, hearing loss, diabetes, Parkinson and Alzheimer1–3. Light-activated interfaces provide a wireless and non-genetic way to modulate neurons with high spatiotemporal resolution, which make them a promising alternative to wired and surgically more invasive electrical stimulation electrodes4,5. -

A Practical Phase Gate for Producing Bell Violations in Majorana Wires
PHYSICAL REVIEW X 6, 021005 (2016) A Practical Phase Gate for Producing Bell Violations in Majorana Wires David J. Clarke, Jay D. Sau, and Sankar Das Sarma Department of Physics, Condensed Matter Theory Center, University of Maryland, College Park, Maryland 20742, USA and Joint Quantum Institute, University of Maryland, College Park, Maryland 20742, USA (Received 9 October 2015; published 8 April 2016) Carrying out fault-tolerant topological quantum computation using non-Abelian anyons (e.g., Majorana zero modes) is currently an important goal of worldwide experimental efforts. However, the Gottesman- Knill theorem [1] holds that if a system can only perform a certain subset of available quantum operations (i.e., operations from the Clifford group) in addition to the preparation and detection of qubit states in the computational basis, then that system is insufficient for universal quantum computation. Indeed, any measurement results in such a system could be reproduced within a local hidden variable theory, so there is no need for a quantum-mechanical explanation and therefore no possibility of quantum speedup [2]. Unfortunately, Clifford operations are precisely the ones available through braiding and measurement in systems supporting non-Abelian Majorana zero modes, which are otherwise an excellent candidate for topologically protected quantum computation. In order to move beyond the classically simulable subspace, an additional phase gate is required. This phase gate allows the system to violate the Bell-like Clauser- Horne-Shimony-Holt (CHSH) inequality that would constrain a local hidden variable theory. In this article, we introduce a new type of phase gate for the already-existing semiconductor-based Majorana wire systems and demonstrate how this phase gate may be benchmarked using CHSH measurements. -

1.07 Quantum Dots: Theory N Vukmirovic´ and L-W Wang, Lawrence Berkeley National Laboratory, Berkeley, CA, USA
1.07 Quantum Dots: Theory N Vukmirovic´ and L-W Wang, Lawrence Berkeley National Laboratory, Berkeley, CA, USA ª 2011 Elsevier B.V. All rights reserved. 1.07.1 Introduction 189 1.07.2 Single-Particle Methods 190 1.07.2.1 Density Functional Theory 191 1.07.2.2 Empirical Pseudopotential Method 193 1.07.2.3 Tight-Binding Methods 194 1.07.2.4 k ? p Method 195 1.07.2.5 The Effect of Strain 198 1.07.3 Many-Body Approaches 201 1.07.3.1 Time-Dependent DFT 201 1.07.3.2 Configuration Interaction Method 202 1.07.3.3 GW and BSE Approach 203 1.07.3.4 Quantum Monte Carlo Methods 204 1.07.4 Application to Different Physical Effects: Some Examples 205 1.07.4.1 Electron and Hole Wave Functions 205 1.07.4.2 Intraband Optical Processes in Embedded Quantum Dots 206 1.07.4.3 Size Dependence of the Band Gap in Colloidal Quantum Dots 208 1.07.4.4 Excitons 209 1.07.4.5 Auger Effects 210 1.07.4.6 Electron–Phonon Interaction 212 1.07.5 Conclusions 213 References 213 1.07.1 Introduction laterally by electrostatic gates or vertically by etch- ing techniques [1,2]. The properties of this type of Since the early 1980s, remarkable progress in technology quantum dots, sometimes termed as electrostatic has been made, enabling the production of nanometer- quantum dots, can be controlled by changing the sized semiconductor structures. This is the length scale applied potential at gates, the choice of the geometry where the laws of quantum mechanics rule and a range of gates, or external magnetic field. -

Electrodynamic Modeling of Quantum Dot Luminescence in Plasmonic Metamaterials † ‡ † ‡ ‡ § Ming Fang,*, , Zhixiang Huang,*, Thomas Koschny, and Costas M
Article pubs.acs.org/journal/apchd5 Electrodynamic Modeling of Quantum Dot Luminescence in Plasmonic Metamaterials † ‡ † ‡ ‡ § Ming Fang,*, , Zhixiang Huang,*, Thomas Koschny, and Costas M. Soukoulis , † Key Laboratory of Intelligent Computing and Signal Processing, Ministry of Education, Anhui University, Hefei 230001, China ‡ Ames Laboratory and Department of Physics and Astronomy, Iowa State University, Ames, Iowa 50011, United States § Institute of Electronic Structure and Laser, FORTH, 71110 Heraklion, Crete, Greece ABSTRACT: A self-consistent approach is proposed to simulate a coupled system of quantum dots (QDs) and metallic metamaterials. Using a four-level atomic system, an artificial source is introduced to simulate the spontaneous emission process in the QDs. We numerically show that the metamaterials can lead to multifold enhancement and spectral narrowing of photoluminescence from QDs. These results are consistent with recent experimental studies. The proposed method represents an essential step for developing and understanding a metamaterial system with gain medium inclusions. KEYWORDS: photoluminescence, plasmonics, metamaterials, quantum dots, finite-different time-domain, spontaneous emission he fields of plasmonic metamaterials have made device design based on quantum electrodynamics. In an active − T spectacular experimental progress in recent years.1 3 medium, the electromagnetic field is treated classically, whereas The metal-based metamaterial losses at optical frequencies atoms are treated quantum mechanically. According to the are unavoidable. Therefore, control of conductor losses is a key current understanding, the interaction of electromagnetic fields challenge in the development of metamaterial technologies. with an active medium can be modeled by a classical harmonic These losses hamper the development of optical cloaking oscillator model and the rate equations of atomic population devices and negative index media. -

Modeling Multiple Quantum Well and Superlattice Solar Cells
Natural Resources, 2013, 4, 235-245 235 http://dx.doi.org/10.4236/nr.2013.43030 Published Online July 2013 (http://www.scirp.org/journal/nr) Modeling Multiple Quantum Well and Superlattice Solar Cells Carlos I. Cabrera1, Julio C. Rimada2, Maykel Courel2,3, Luis Hernandez4,5, James P. Connolly6, Agustín Enciso4, David A. Contreras-Solorio4 1Department of Physics, University of Pinar del Río, Pinar del Río, Cuba; 2Solar Cell Laboratory, Institute of Materials Science and Technology (IMRE), University of Havana, Havana, Cuba; 3Higher School in Physics and Mathematics, National Polytechnic Insti- tute, Mexico City, Mexico; 4Academic Unit of Physics, Autonomous University of Zacatecas, Zacatecas, México; 5Faculty of Phys- ics, University of Havana, La Habana, Cuba; 6Nanophotonics Technology Center, Universidad Politécnica de Valencia, Valencia, Spain. Email: [email protected] Received January 23rd, 2013; revised May 14th, 2013; accepted May 27th, 2013 Copyright © 2013 Carlos I. Cabrera et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The inability of a single-gap solar cell to absorb energies less than the band-gap energy is one of the intrinsic loss mechanisms which limit the conversion efficiency in photovoltaic devices. New approaches to “ultra-high” efficiency solar cells include devices such as multiple quantum wells (QW) and superlattices (SL) systems in the intrinsic region of a p-i-n cell of wider band-gap energy (barrier or host) semiconductor. These configurations are intended to extend the absorption band beyond the single gap host cell semiconductor. -

Quantum Computation with Two-Dimensional Graphene
Quantum computation with two-dimensional graphene quantum dots* Jason Lee(李杰森), Zhi-Bing Li(李志兵), and Dao-Xin Yao (姚道新)†† State Key Laboratory of Optoelectronic Materials and Technologies, School of Physics and Engineering, Sun Yat-sen University, Guangzhou 510275, China Keywords: graphene, quantum dot, quantum computation, Kagome lattice PACS: 73.22.Pr, 73.21.La, 73.22.–f, 74.25.Jb Abstract We study an array of graphene nano sheets that form a two-dimensional S = 1/2 Kagome spin lattice used for quantum computation. The edge states of the graphene nano sheets are used to form quantum dots to confine electrons and perform the computation. We propose two schemes of bang-bang control to combat decoherence and realize gate operations on this array of quantum dots. It is shown that both schemes contain a great amount of information for quantum computation. The corresponding gate operations are also proposed. 1. Introduction There has been increasing interest in grapheme since its discovery. [1−3] It has shown excellent electronic[4,5] and mechanical [6,7] properties and is also a promising candidate for biosensors.[8,9] Before this amazing discovery, Wallace had studied the band structure of graphite and found a linear dispersion around the Dirac point in the Brillouin zone. [10]Much research has been done on this linear dispersion and in particular on the transport properties of graphene. [11−13] Nakada and Fujita studied the edge state and the nano size effect of graphene, and found that the charge can be localized in the zigzag edge[14] to form quantum dots (QDs).