Citral-Containing Essential Oils As Potential Tyrosinase Inhibitors: a Bio-Guided Fractionation Approach
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Following Carcinogenic Essential Oils Should Not Be Used In
Aromatherapy Undiluted- Safety and Ethics Copyright © Tony Burfield and Sylla Sheppard-Hanger (2005) [modified from a previous article “A Brief Safety Guidance on Essential Oils” written for IFA, Sept 2004]. Intro In the last 20 years aromatherapy has spread its influence to the household, toiletries and personal care areas: consumer products claiming to relax or invigorate our psyche’s have invaded our bathrooms, kitchen and living room areas. The numbers of therapists using essential oils in Europe and the USA has grown from a handful in the early 1980’s to thousands now worldwide. We have had time to add to our bank of knowledge on essential oils from reflecting on many decades of aromatherapeutic development and history, the collection of anecdotal information from practicing therapists, as well as from clinical & scientific investigations. We have also had enough time to consider the risks in employing essential oils in therapy. In the last twenty years, many more people have had accidents, been ‘burnt’, developed rashes, become allergic, and become sensitized to our beloved tools. Why is this? In this paper, we hope to shed light on this issue, clarify current safety findings, and discuss how Aromatherapists and those in the aromatherapy trade (suppliers, spas, etc.) can interpret this data for continued safe practice. After a refresher on current safety issues including carcinogenic and toxic oils, irritant and photo-toxic oils, we will look at allergens, oils without formal testing, pregnancy issues and medication interactions. We will address the increasing numbers of cases of sensitization and the effect of diluting essential oils. -

Five Hundred Plant Species in Gunung Halimun Salak National Park, West Java a Checklist Including Sundanese Names, Distribution and Use
Five hundred plant species in Gunung Halimun Salak National Park, West Java A checklist including Sundanese names, distribution and use Hari Priyadi Gen Takao Irma Rahmawati Bambang Supriyanto Wim Ikbal Nursal Ismail Rahman Five hundred plant species in Gunung Halimun Salak National Park, West Java A checklist including Sundanese names, distribution and use Hari Priyadi Gen Takao Irma Rahmawati Bambang Supriyanto Wim Ikbal Nursal Ismail Rahman © 2010 Center for International Forestry Research. All rights reserved. Printed in Indonesia ISBN: 978-602-8693-22-6 Priyadi, H., Takao, G., Rahmawati, I., Supriyanto, B., Ikbal Nursal, W. and Rahman, I. 2010 Five hundred plant species in Gunung Halimun Salak National Park, West Java: a checklist including Sundanese names, distribution and use. CIFOR, Bogor, Indonesia. Photo credit: Hari Priyadi Layout: Rahadian Danil CIFOR Jl. CIFOR, Situ Gede Bogor Barat 16115 Indonesia T +62 (251) 8622-622 F +62 (251) 8622-100 E [email protected] www.cifor.cgiar.org Center for International Forestry Research (CIFOR) CIFOR advances human wellbeing, environmental conservation and equity by conducting research to inform policies and practices that affect forests in developing countries. CIFOR is one of 15 centres within the Consultative Group on International Agricultural Research (CGIAR). CIFOR’s headquarters are in Bogor, Indonesia. It also has offices in Asia, Africa and South America. | iii Contents Author biographies iv Background v How to use this guide vii Species checklist 1 Index of Sundanese names 159 Index of Latin names 166 References 179 iv | Author biographies Hari Priyadi is a research officer at CIFOR and a doctoral candidate funded by the Fonaso Erasmus Mundus programme of the European Union at Southern Swedish Forest Research Centre, Swedish University of Agricultural Sciences. -

Effect of Alkali Carbonate/Bicarbonate on Citral Hydrogenation Over Pd/Carbon Molecular Sieves Catalysts in Aqueous Media
Modern Research in Catalysis, 2016, 5, 1-10 Published Online January 2016 in SciRes. http://www.scirp.org/journal/mrc http://dx.doi.org/10.4236/mrc.2016.51001 Effect of Alkali Carbonate/Bicarbonate on Citral Hydrogenation over Pd/Carbon Molecular Sieves Catalysts in Aqueous Media Racharla Krishna, Chowdam Ramakrishna, Keshav Soni, Thakkallapalli Gopi, Gujarathi Swetha, Bijendra Saini, S. Chandra Shekar* Defense R & D Establishment, Gwalior, India Received 18 November 2015; accepted 5 January 2016; published 8 January 2016 Copyright © 2016 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract The efficient citral hydrogenation was achieved in aqueous media using Pd/CMS and alkali addi- tives like K2CO3. The alkali concentrations, reaction temperature and the Pd metal content were optimized to enhance the citral hydrogenation under aqueous media. In the absence of alkali, ci- tral hydrogenation was low and addition of alkali promoted to ~92% hydrogenation without re- duction in the selectivity to citronellal. The alkali addition appears to be altered the palladium sites. The pore size distribution reveals that the pore size of these catalysts is in the range of 0.96 to 0.7 nm. The palladium active sites are also quite uniform based on the TPR data. The catalytic parameters are correlated well with the activity data. *Corresponding author. How to cite this paper: Krishna, R., Ramakrishna, C., Soni, K., Gopi, T., Swetha, G., Saini, B. and Shekar, S.C. (2016) Effect of Alkali Carbonate/Bicarbonate on Citral Hydrogenation over Pd/Carbon Molecular Sieves Catalysts in Aqueous Media. -

Citral (Microencapsulated)
NTP TECHNICAL REPORT ON THE TOXICOLOGY AND CARCINOGENESIS STUDIES OF CITRAL (MICROENCAPSULATED) (CAS NO. 5392-40-5) IN F344/N RATS AND B6C3F1 MICE (FEED STUDIES) NATIONAL TOXICOLOGY PROGRAM P.O. Box 12233 Research Triangle Park, NC 27709 January 2003 NTP TR 505 NIH Publication No. 03-4439 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health FOREWORD The National Toxicology Program (NTP) is made up of four charter agencies of the U.S. Department of Health and Human Services (DHHS): the National Cancer Institute (NCI), National Institutes of Health; the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health; the National Center for Toxicological Research (NCTR), Food and Drug Administration; and the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. In July 1981, the Carcinogenesis Bioassay Testing Program, NCI, was transferred to the NIEHS. The NTP coordinates the relevant programs, staff, and resources from these Public Health Service agencies relating to basic and applied research and to biological assay development and validation. The NTP develops, evaluates, and disseminates scientific information about potentially toxic and hazardous chemicals. This knowledge is used for protecting the health of the American people and for the primary prevention of disease. The studies described in this Technical Report were performed under the direction of the NIEHS and were conducted in compliance with NTP laboratory health and safety requirements and must meet or exceed all applicable federal, state, and local health and safety regulations. Animal care and use were in accordance with the Public Health Service Policy on Humane Care and Use of Animals. -

Olfactometer Screening of Repellent Essential Oils Against the Pollen Beetle (Meligethes Spp.)
Forschungsinstitut für biologischen Landbau Institut de recherche de l’agriculture biologique Research Institute of Organic Agriculture Istituto di ricerche dell’agricoltura biologica Instituto de investigaciones para la agricultura orgánica C. Daniel EXCELLENCE FOR SUSTAINABILITY Research Institute of Organic Agriculture, Ackerstrasse 21, CH-5070 Frick, Switzerland, [email protected] Olfactometer screening of repellent essential oils against the pollen beetle (Meligethes spp.). Odour 1 Odour 2 Air flow Introduction Organic agricultural methods to control pol- in spring. Non-host odors can have repellent len beetle (Meligethes spp.) are limited and effects on pollen beetles (Maucheline 2005). alternatives are needed. The beetles use olfac- We compared the repellent effects of 15 differ- tory cues to locate oilseed rape fields during immigration ent essential oils using a Y-tube-olfactometer. Material and methods Tested essential oils: � Y-tube-olfactometer described by Belz et al. 2013. � Cornmint (Mentha arvensis), � Essential oils diluted 1:10 in acetone; 40 µl applied on filter paper. � Orange (Citrus sinensis), � Filter papers + a flower cluster of oilseed rape were placed in the � Wintergreen (Gaultheria procumbens), odour containers of the olfactometer (control treatment: filter papers � Lemongrass (Cymbopogon flexuosus), with acetone +flower). � Eucalyptus (Eucalyptus globulus), � Beetles were released individually into the olfactometer. � Fir needle (Abies sibirica), � The beetles’ choices were recorded. � Lemon (Citrus limon), � Six replicates with six beetles each were conducted. � Tea-tree (Melaleuca alternifolia), � Repellency values (RV) = nb of beetles on the untreated flower / total � Clove (Syzygium aromaticum), nb of beetles. � Star anise (Illicium verrum), � Wilcoxon signed rank test to test whether mean RV significantly differ- � Grapefruit white (Citrus paradisi), ent from 0.5. -

In Vitro Antibacterial Activity of 34 Plant Essential Oils Against Alternaria Alternata
E3S Web o f Conferences 136, 06006 (2019) https://doi.org/10.1051/e3sconf/20191360 6006 ICBTE 2019 In vitro antibacterial activity of 34 plant essential oils against Alternaria alternata Qiyu Lu1, Ji Liu2, Caihong Tu2, Juan Li3, Chunlong Lei3, Qiliang Guo2, Zhengzhou Zhang2, Wen Qin1* 1College of Food Science and Technology, Sichuan Agricultural University, Ya'an, Sichuan, 625014, China 2Institute of Food Science and Technology, Chengdu Academy of Agricultural and Forestry Science, Chengdu, Sichuan, 611130, China 3 Institute of Animal Science, Chengdu Academy of Agricultural and Forestry Science, Chengdu, Sichuan, 611130, China Abstract. To determine the antibacterial effect of 34 plant essential oils on Alternaria alternata, 34 plant essential oils such as asarum essential oil, garlic essential oil, and mustard essential oil are used as inhibition agents to isolate A. alternata from citrus as indicator bacteria, through the bacteriostasis test and drug susceptibility test, the types of essential oils with the best inhibitory effect were screened and their concentration was determined. The results showed that the best inhibition effect was mustard essential oil with a minimum inhibitory concentration of 250 μl/L and a minimum bactericidal concentration of 250 μl/L. Followed by the Litsea cubeba essential oil and basil oil, the minimum inhibitory concentration is 500 μl/L. 1 Introduction quality and safety. A. alternata is the main fungus causing post-harvest disease of Pitaya, and the Citrus is a general term for orange, mandarin, orange, antibacterial effect of using cinnamon oil and clove oil is kumquat, pomelo, and medlar. Citrus is the world's good [10]. Clove oil can effectively inhibit the growth of largest fruit, rich in sugar, organic acids, mineral A. -

The Litsea Genome and the Evolution of the Laurel Family
The Litsea genome and the evolution of the laurel family Chen et al 1 Supplementary Note 1. Sample preparation for Litsea cubeba genome sequencing For genome sequencing, we collected buds of L. cubeba. Genomic DNA was extracted using a modified cetyltrimethylammonium bromide (CTAB) protocol. For transcriptome analysis, we collected leaves, flowers, and roots from L. cubeba in Zhejiang Province, China, using a karyotype of 2n = 24 (Supplementary Figure 2a). Genome sizes can be determined from the total number of k-mers, divided by the peak value of the k-mer distribution1. To estimate the genome size of L. cubeba, we used a 350 bp pair-end library with 93.08 Gb high-quality reads to calculate the distribution of k-mer values, and found the main peak to be 54 (Supplementary Figure 2b). We estimated the L. cubeba genome size as 1370.14 Mbp, with a 1% heterozygosity rate and a 70.59% repeat sequence, based on an analysis of k-mer-numbers/depths. We used k-mer 41 to obtain a preliminary assembly of L. cubeba, with a scaffold N50 size of 776 bp and a corresponding contig N50 size of 591 bp. Supplementary Note 2. Whole genome duplication analysis in Laurales The KS peaks for WGDs in L. cubeba are both younger (smaller KS values) than the orthologous KS peak between L. cubeba and V. vinifera, implying that the two WGD events are specific to Magnoliids. To compare the WGD peaks of L. cubeba and the speciation events in the lineage of Magnoliids, we performed relative rate tests and corrected orthologous KS peaks between L. -

Herbs, Spices and Essential Oils
Printed in Austria V.05-91153—March 2006—300 Herbs, spices and essential oils Post-harvest operations in developing countries UNITED NATIONS INDUSTRIAL DEVELOPMENT ORGANIZATION Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria Telephone: (+43-1) 26026-0, Fax: (+43-1) 26926-69 UNITED NATIONS FOOD AND AGRICULTURE E-mail: [email protected], Internet: http://www.unido.org INDUSTRIAL DEVELOPMENT ORGANIZATION OF THE ORGANIZATION UNITED NATIONS © UNIDO and FAO 2005 — First published 2005 All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of material in this information product for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission should be addressed to: - the Director, Agro-Industries and Sectoral Support Branch, UNIDO, Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria or by e-mail to [email protected] - the Chief, Publishing Management Service, Information Division, FAO, Viale delle Terme di Caracalla, 00100 Rome, Italy or by e-mail to [email protected] The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the United Nations Industrial Development Organization or of the Food and Agriculture Organization of the United Nations concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Ethnopharmacological Properties and Medicinal Uses of Litsea Cubeba
plants Review Ethnopharmacological Properties and Medicinal Uses of Litsea cubeba Madhu Kamle 1, Dipendra K. Mahato 2, Kyung Eun Lee 3, Vivek K. Bajpai 4, Padam Raj Gajurel 1, Kang Sang Gu 3,5,* and Pradeep Kumar 1,* 1 Department of Forestry, North Eastern Regional Institute of Science and Technology, Nirjuli 791109, India; [email protected] (M.K.); [email protected] (P.R.G.) 2 School of Exercise and Nutrition Sciences, Deakin University, Burwood, VIC 3125, Australia; [email protected] 3 Molecular Genetics Lab, Department of Biotechnology, Yeungnam University, Gyeongsan, Gyeongbuk 38541, Korea; [email protected] 4 Department of Energy and Material Engineering, Dongguk University-Seoul, Seoul 04620, Korea; [email protected] 5 Stemforce, 302 Institute of Industrial Technology, Yeungnam University, Gyeongsan, Gyeongbuk 38541, Korea; [email protected] * Correspondence: [email protected] (K.S.G.); [email protected] (P.K.) Received: 8 May 2019; Accepted: 30 May 2019; Published: 1 June 2019 Abstract: The genus Litsea is predominant in tropical and subtropical regions of India, China, Taiwan, and Japan. The plant possesses medicinal properties and has been traditionally used for curing various gastro-intestinal ailments (e.g., diarrhea, stomachache, indigestion, and gastroenteritis) along with diabetes, edema, cold, arthritis, asthma, and traumatic injury. Besides its medicinal properties, Litsea is known for its essential oil, which has protective action against several bacteria, possesses antioxidant and antiparasitic properties, exerts acute and genetic toxicity as well as cytotoxicity, and can even prevent several cancers. Here we summarize the ethnopharmacological properties, essentials oil, medicinal uses, and health benefits of an indigenous plant of northeast India, emphasizing the profound research to uplift the core and immense potential present in the conventional medicine of the country. -
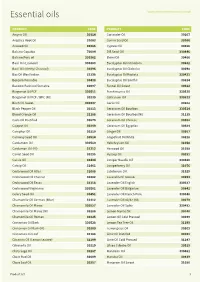
Essential Oils
Essential oils www.inoviainternational.co.uk PRODUCT CODE PRODUCT CODE Amyris Oil 30314 Coriander Oil 30107 Angelica Root Oil 31063 Cumin Seed Oil 30266 Aniseed Oil 30385 Cypress Oil 30256 Balsam Copaiba 70094 Dill Seed Oil 330446 Balsam Peru oil 330362 Elemi Oil 30466 Basil Oil (Linalool) 330483 Eucalyptus Oil Citriodora 30632 Basil Oil (Methyl Chavicol) 30296 Eucalyptus Oil Globulus 30298 Bay Oil West Indian 31398 Eucalyptus Oil Radiata 330421 Benzoin Pourable 30438 Eucalyptus Oil Smithii 30814 Benzoin Resinoid Sumatra 60097 Fennel Oil Sweet 30322 Bergamot Oil FCF 330511 Frankincense Oil 330525 Bergamot Oil FCF / BPC (NI) 30239 Galbanum Oil 330523 Birch Oil Sweet 330437 Garlic Oil 30102 Black Pepper Oil 30313 Geranium Oil Bourbon 330514 Blood Orange Oil 32188 Geranium Oil Bourbon(NI) 31135 Cade Oil Rectified 30679 Geranium Oil Chinese 30302 Cajeput Oil 30249 Geranium Oil Egyptian 30384 Camphor Oil 30110 Ginger Oil 30367 Caraway Seed Oil 30514 Grapefruit Oil White 30228 Cardamom Oil 330510 Helichrysum Oil 31058 Cardamon Oil (NI) 31212 Ho wood Oil 31038 Carrot Seed Oil 30295 Hyssop Oil 30281 Cassia Oil 30434 Juniper Needle Oil 330480 Catnip Oil 31461 Juniperberry Oil 31076 Cedarwood Oil Atlas 31088 Labdanum Oil 31929 Cedarwood Oil Chinese 30282 Lavandin Oil Grosso 30293 Cedarwood Oil Texas 31118 Lavender Oil English 330537 Cedarwood Virginiana 330501 Lavender Oil Bulgarian 30841 Celery Seed Oil 30451 Lavender Oil French Pure 330348 Chamomile Oil German (blue) 31412 Lavender Oil 40/42 (NI) 30279 Chamomile Oil Maroc 330527 Lavender Oil Spike -

Use of Essential Oils of the Genus Citrus As Biocidal Agents
American Journal of Plant Sciences, 2014, 5, 299-305 Published Online February 2014 (http://www.scirp.org/journal/ajps) http://dx.doi.org/10.4236/ajps.2014.53041 Use of Essential Oils of the Genus Citrus as Biocidal Agents Marcos S. Gomes1, Maria das G. Cardoso1*, Maurilio J. Soares2, Luís R. Batista3, Samísia M. F. Machado4, Milene A. Andrade1, Camila M. O. de Azeredo2, Juliana Maria Valério Resende3, Leonardo M. A. Rodrigues3 1Department of Chemistry, Federal University of Lavras, Lavras, Brazil; 2Laboratory of Cell Biology, Instituto Carlos Cha- gas/Fiocruz, Curitiba, Brasil; 3Department of Food Science, Federal University of Lavras, Lavras, Brazil; 4Department of Chemistry, Federal University of Sergipe, São Cristóvão, Brazil. Email: *[email protected] Received November 26th, 2013; revised December 28th, 2013; accepted January 18th, 2014 Copyright © 2014 Marcos S. Gomes et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accor- dance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Marcos S. Gomes et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian. ABSTRACT In this study, the essential oils extracted from the peels of Citrus aurantifolia, Citrus limon and Citrus sinensis were chemically characterized and quantified. These essential oils and their standards limonene, citral and li- monene + citral were evaluated (at concentrations ranging from 500 to 3.91 mL·mL−1) regarding their anti-try- panosome, antifungal and antibacterial activities. -

Citral Cas N°:5392-40-5
OECD SIDS CITRAL FOREWORD INTRODUCTION CITRAL CAS N°:5392-40-5 UNEP PUBLICATIONS 1 OECD SIDS CITRAL SIDS Initial Assessment Report for 13th SIAM (Switzerland, November 6-9, 2001) Chemical Name : Citral CAS No: 5392-40-5 Sponsor Country: Japan National SIDS Contact Point in Sponsor Country: Mr. Yasuhisa Kawamura, Ministry of Foreign Affairs, Japan History: This SIAR was discussed at SIAM11 (USA, January, 2001) at which the human health assessment and its conclusion were accepted. The environmental assessment was revised after SIAM11 and the revised SIAR was discussed and finalised at SIAM13. Tests: No testing ( ) Testing (x) Vapor pressure, log Pow, Water solubility, Hydrolysis and Photolysis, Biodegradation, Environmental fate, Acute toxicity to fish, daphnia and algae, Chronic toxicity to daphnia, Preliminary reproduction toxicity Comment: 2 UNEP PUBLICATIONS OECD SIDS CITRAL SIDS INITIAL ASSESSMENT PROFILE CAS No. 5392-40-5 Chemical Name Citral Structural Formula O C10H16O RECOMMENDATIONS The chemical is currently of low priority for further work. SUMMARY CONCLUSIONS OF THE SIAR Human Health Citral was rapidly absorbed from the gastro -intestinal tract. Much of an applied dermal dose was lost due to its extreme volatility, but the citral remaining on the skin was fairly well absorbed. Citral was rapidly metabolized and excreted as metabolites. Urine was the major route of elimination. Acute toxicity of this chemical is low in rodents because the oral or dermal LD50 values were more than 1000 mg/kg. This chemical is irritating to skin and not irritating to eyes in rabbits. There is some evidence that this chemical is a human skin sensitizer.