First Use of Anatomical Networks to Study Modularity and Integration Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ultrasound Evaluation of the Abductor Hallucis Muscle: Reliability Study Alyse FM Cameron, Keith Rome and Wayne a Hing*
Journal of Foot and Ankle Research BioMed Central Research Open Access Ultrasound evaluation of the abductor hallucis muscle: Reliability study Alyse FM Cameron, Keith Rome and Wayne A Hing* Address: AUT University, School of Rehabilitation & Occupation Studies, Health & Rehabilitation Research Centre, Private Bag 92006, Auckland, 1142, New Zealand Email: Alyse FM Cameron - [email protected]; Keith Rome - [email protected]; Wayne A Hing* - [email protected] * Corresponding author Published: 25 September 2008 Received: 29 May 2008 Accepted: 25 September 2008 Journal of Foot and Ankle Research 2008, 1:12 doi:10.1186/1757-1146-1-12 This article is available from: http://www.jfootankleres.com/content/1/1/12 © 2008 Cameron et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: The Abductor hallucis muscle (AbdH) plays an integral role during gait and is often affected in pathological foot conditions. The aim of this study was to evaluate the within and between-session intra-tester reliability using diagnostic ultrasound of the dorso-plantar thickness, medio-lateral width and cross-sectional area, of the AbdH in asymptomatic adults. Methods: The AbdH muscles of thirty asymptomatic subjects were imaged and then measured using a Philips HD11 Ultrasound machine. Interclass correlation coefficients (ICC) with 95% confidence intervals (CI) were used to calculate both within and between session intra-tester reliability. Results: The within-session reliability results demonstrated for dorso-plantar thickness an ICC of 0.97 (95% CI: 0.99–0.99); medio-lateral width an ICC: of 0.97 (95% CI: 0.92–0.97) and cross- sectional area an ICC of 0.98 (95% CI: 0.98–0.99). -
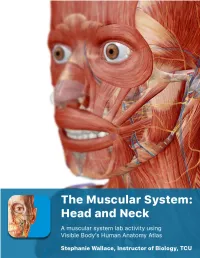
The Muscular System Views
1 PRE-LAB EXERCISES Before coming to lab, get familiar with a few muscle groups we’ll be exploring during lab. Using Visible Body’s Human Anatomy Atlas, go to the Views section. Under Systems, scroll down to the Muscular System views. Select the view Expression and find the following muscles. When you select a muscle, note the book icon in the content box. Selecting this icon allows you to read the muscle’s definition. 1. Occipitofrontalis (epicranius) 2. Orbicularis oculi 3. Orbicularis oris 4. Nasalis 5. Zygomaticus major Return to Muscular System views, select the view Head Rotation and find the following muscles. 1. Sternocleidomastoid 2. Scalene group (anterior, middle, posterior) 2 IN-LAB EXERCISES Use the following modules to guide your exploration of the head and neck region of the muscular system. As you explore the modules, locate the muscles on any charts, models, or specimen available. Please note that these muscles act on the head and neck – those that are located in the neck but act on the back are in a separate section. When reviewing the action of a muscle, it will be helpful to think about where the muscle is located and where the insertion is. Muscle physiology requires that a muscle will “pull” instead of “push” during contraction, and the insertion is the part that will move. Imagine that the muscle is “pulling” on the bone or tissue it is attached to at the insertion. Access 3D views and animated muscle actions in Visible Body’s Human Anatomy Atlas, which will be especially helpful to visualize muscle actions. -

Axis Scientific Skull with Muscle Origins and Insertions A-108851
Axis Scientific Skull with Muscle Origins and Insertions A-108851 *Muscle Origins = RED Anterior View Occipital Bone Posterior View *Muscle Insertions = BLUE Posterior Cerebral Artery Frontal Bone Basilar Artery Pontine Arteries Parietal Bone Nasal Bone Temporal Bone Sphenoid Bone Temporalis Parietal Bone Occipitofrontalis Occipital Bone Lacrimal Bone Sternocleidomastoid Trapezius Temporalis Semispinalis Capitis Temporal Bone Corrugator Supercilii Splenius Capitis Longissimus Capitis Orbicularis Oculi Obliquus Capitis Superior Temporal Basilar Artery Bone Procerus Rectis Capitis C1 Posterior Major Vertebral Artery Levator Labii Superioris C2 Alaeque Nasi Rectis Capitis Posterior Minor Sphenoid Levator Labii Superioris Posterior Digastric Zygomatic Bone Bone C3 Nasalis (Transverse Part) Rectis Capitis C4 Masseter Lateralis Spinal Nerve Zygomaticus Major C5 Zygomatic Medial Pterygoid Bone Zygomaticus Minor C6 Temporalis Mylohyoid C7 Mandible Levator Anguli Oris Vomer Nasalis (Alar Part) Spinal Nerve Spinal Cord Maxilla Orbicularis Oris Depressor Septi Nasi Masseter Medial Superior Masseter Mandible Orbicularis Oris Constrictor Pterygoid Inferior View Styloglossus Platysma Mylohyoid Depressor Stylohyoid Anguli Oris Anterior Stylopharyngeus Spinal Nerve Depressor Digastric Labii Inferioris Posterior Geniohyoid Digastric Vertebral Artery Mentalis Rectis Capitis Genioglossus Lateralis Rectis Capitis Buccinator Mandible Posterior Major Rectis Capitis Posterior Minor Frontal Bone Corrugator Supercilii Orbicularis Oculi Lacrimal Bone Depressor -

Questions on Human Anatomy
Standard Medical Text-books. ROBERTS’ PRACTICE OF MEDICINE. The Theory and Practice of Medicine. By Frederick T. Roberts, m.d. Third edi- tion. Octavo. Price, cloth, $6.00; leather, $7.00 Recommended at University of Pennsylvania. Long Island College Hospital, Yale and Harvard Colleges, Bishop’s College, Montreal; Uni- versity of Michigan, and over twenty other medical schools. MEIGS & PEPPER ON CHILDREN. A Practical Treatise on Diseases of Children. By J. Forsyth Meigs, m.d., and William Pepper, m.d. 7th edition. 8vo. Price, cloth, $6.00; leather, $7.00 Recommended at thirty-five of the principal medical colleges in the United States, including Bellevue Hospital, New York, University of Pennsylvania, and Long Island College Hospital. BIDDLE’S MATERIA MEDICA. Materia Medica, for the Use of Students and Physicians. By the late Prof. John B Biddle, m.d., Professor of Materia Medica in Jefferson Medical College, Phila- delphia. The Eighth edition. Octavo. Price, cloth, $4.00 Recommended in colleges in all parts of the UnitedStates. BYFORD ON WOMEN. The Diseases and Accidents Incident to Women. By Wm. H. Byford, m.d., Professor of Obstetrics and Diseases of Women and Children in the Chicago Medical College. Third edition, revised. 164 illus. Price, cloth, $5.00; leather, $6.00 “ Being particularly of use where questions of etiology and general treatment are concerned.”—American Journal of Obstetrics. CAZEAUX’S GREAT WORK ON OBSTETRICS. A practical Text-book on Midwifery. The most complete book now before the profession. Sixth edition, illus. Price, cloth, $6.00 ; leather, $7.00 Recommended at nearly fifty medical schools in the United States. -

The Articulatory System Chapter 6 Speech Science/ COMD 6305 UTD/ Callier Center William F. Katz, Ph.D
The articulatory system Chapter 6 Speech Science/ COMD 6305 UTD/ Callier Center William F. Katz, Ph.D. STRUCTURE/FUNCTION VOCAL TRACT CLASSIFICATION OF CONSONANTS AND VOWELS MORE ON RESONANCE ACOUSTIC ANALYSIS/ SPECTROGRAMS SUPRSEGMENTALS, COARTICULATION 1 Midsagittal dissection From Kent, 1997 2 Oral Cavity 3 Oral Structures – continued • Moistened by saliva • Lined by mucosa • Saliva affected by meds 4 Tonsils • PALATINE* (laterally – seen in oral periph • LINGUAL (inf.- root of tongue) • ADENOIDS (sup.) [= pharyngeal] • Palatine, lingual tonsils are larger in children • *removed in tonsillectomy 5 Adenoid Facies • Enlargement from infection may cause problems (adenoid facies) • Can cause problems with nasal sounds or voicing • Adenoidectomy; also tonsillectomy (for palatine tonsils) 6 Adenoid faces (example) 7 Oral structures - frenulum Important component of oral periphery exam Lingual frenomy – for ankyloglossia “tongue-tie” Some doctors will snip for infants, but often will loosen by itself 8 Hard Palate Much variability in palate shape and height Very high vault 9 Teeth 10 Dentition - details Primary (deciduous, milk teeth) Secondary (permanent) n=20: n=32: ◦ 2 incisor ◦ 4 incisor ◦ 1 canine ◦ 2 canine ◦ 2 molar ◦ 4 premolar (bicuspid) Just for “fun” – baby ◦ 6 molar teeth pushing in! NOTE: x 2 for upper and lower 11 Types of malocclusion • Angle’s classification: • I, II, III • Also, individual teeth can be misaligned (e.g. labioversion) Also “Neutrocclusion/ distocclusion/mesiocclusion” 12 Dental Occlusion –continued Other terminology 13 Mandible Action • Primary movements are elevation and depression • Also…. protrusion/retraction • Lateral grinding motion 14 Muscles of Jaw Elevation Like alligators, we are much stronger at jaw elevation (closing to head) than depression 15 Jaw Muscles ELEVATORS DEPRESSORS •Temporalis ✓ •Mylohyoid ✓ •Masseter ✓ •Geniohyoid✓ •Internal (medial) Pterygoid ✓ •Anterior belly of the digastric (- Kent) •Masseter and IP part of “mandibular sling” •External (lateral) pterygoid(?)-- also protrudes and rocks side to side. -

Anatomical Study of Minimally Invasive Lateral Release
FAIXXX10.1177/1071100720920863Foot & Ankle InternationalDalmau-Pastor et al 920863research-article2020 Article Foot & Ankle International® 1 –9 Anatomical Study of Minimally Invasive © The Author(s) 2020 Article reuse guidelines: sagepub.com/journals-permissions Lateral Release Techniques for Hallux DOI:https://doi.org/10.1177/1071100720920863 10.1177/1071100720920863 Valgus Treatment journals.sagepub.com/home/fai Miki Dalmau-Pastor, PhD1,2 , Francesc Malagelada, MD, PhD1,2,3, Guillaume Cordier, MD2,4, Jorge Javier Del Vecchio, MD, MBA2,5,6 , Mauricio Esteban Ghioldi, MD7, and Jordi Vega, MD1,2,8 Abstract Background: Lateral release (LR) for the treatment of hallux valgus is a routinely performed technique, either by means of open or minimally invasive (MI) surgery. Despite this, there is no available evidence of the efficacy and safety of MI lateral release. Our aim was to study 2 popular techniques for MI LR in cadavers by subsequently dissecting the released anatomical structures. Methods: Twenty-two cadaveric feet were included in the study and allocated into 2 groups, 1 for each procedure: 1 group underwent a MI adductor tendon release (AR), and in the other group, an extensive percutaneous lateral release (EPLR) (adductor tendon, suspensory ligament, phalanx-sesamoid ligament, lateral head of flexor hallucis brevis, and deep transverse metatarsal ligament) was performed. Anatomical dissection was performed to identify neurovascular injuries and to verify the released structures. Results: Both techniques demonstrated to be effective in reproducing a MI LR. A satisfactory release of the adductor tendon was achieved equally in both techniques (P = .85), being partial in most EPLR cases and full in the majority of AR cases. -

Head & Neck Muscle Table
Robert Frysztak, PhD. Structure of the Human Body Loyola University Chicago Stritch School of Medicine HEAD‐NECK MUSCLE TABLE PROXIMAL ATTACHMENT DISTAL ATTACHMENT MUSCLE INNERVATION MAIN ACTIONS BLOOD SUPPLY MUSCLE GROUP (ORIGIN) (INSERTION) Anterior floor of orbit lateral to Oculomotor nerve (CN III), inferior Abducts, elevates, and laterally Inferior oblique Lateral sclera deep to lateral rectus Ophthalmic artery Extra‐ocular nasolacrimal canal division rotates eyeball Inferior aspect of eyeball, posterior to Oculomotor nerve (CN III), inferior Depresses, adducts, and laterally Inferior rectus Common tendinous ring Ophthalmic artery Extra‐ocular corneoscleral junction division rotates eyeball Lateral aspect of eyeball, posterior to Lateral rectus Common tendinous ring Abducent nerve (CN VI) Abducts eyeball Ophthalmic artery Extra‐ocular corneoscleral junction Medial aspect of eyeball, posterior to Oculomotor nerve (CN III), inferior Medial rectus Common tendinous ring Adducts eyeball Ophthalmic artery Extra‐ocular corneoscleral junction division Passes through trochlea, attaches to Body of sphenoid (above optic foramen), Abducts, depresses, and medially Superior oblique superior sclera between superior and Trochlear nerve (CN IV) Ophthalmic artery Extra‐ocular medial to origin of superior rectus rotates eyeball lateral recti Superior aspect of eyeball, posterior to Oculomotor nerve (CN III), superior Elevates, adducts, and medially Superior rectus Common tendinous ring Ophthalmic artery Extra‐ocular the corneoscleral junction division -

Contents VII
Contents VII Contents Preface .............................. V 3.2 Supply of the Connective Tissue ....... 28 List of Abbreviations ................... VI Diffusion ......................... 28 Picture Credits ........................ VI Osmosis .......................... 29 3.3 The “Creep” Phenomenon ............ 29 3.4 The Muscle ....................... 29 Part A Muscle Chains 3.5 The Fasciae ....................... 30 Philipp Richter Functions of the Fasciae .............. 30 Manifestations of Fascial Disorders ...... 30 Evaluation of Fascial Tensions .......... 31 1 Introduction ..................... 2 Causes of Musculoskeletal Dysfunctions .. 31 1.1 The Significance of Muscle Chains Genesis of Myofascial Disorders ........ 31 in the Organism ................... 2 Patterns of Pain .................... 32 1.2 The Osteopathy of Dr. Still ........... 2 3.6 Vegetative Innervation of the Organs ... 34 1.3 Scientific Evidence ................. 4 3.7 Irvin M. Korr ...................... 34 1.4 Mobility and Stability ............... 5 Significance of a Somatic Dysfunction in the Spinal Column for the Entire Organism ... 34 1.5 The Organism as a Unit .............. 6 Significance of the Spinal Cord ......... 35 1.6 Interrelation of Structure and Function .. 7 Significance of the Autonomous Nervous 1.7 Biomechanics of the Spinal Column and System .......................... 35 the Locomotor System .............. 7 Significance of the Nerves for Trophism .. 35 .............. 1.8 The Significance of Homeostasis ....... 8 3.8 Sir Charles Sherrington 36 Inhibition of the Antagonist or Reciprocal 1.9 The Nervous System as Control Center .. 8 Innervation (or Inhibition) ............ 36 1.10 Different Models of Muscle Chains ..... 8 Post-isometric Relaxation ............. 36 1.11 In This Book ...................... 9 Temporary Summation and Local, Spatial Summation .................. 36 Successive Induction ................ 36 ......... 2ModelsofMyofascialChains 10 3.9 Harrison H. Fryette ................. 37 2.1 Herman Kabat 1950: Lovett’s Laws ..................... -

Hallux Varus As Complication of Foot Compartment Syndrome
The Journal of Foot & Ankle Surgery 50 (2011) 504–506 Contents lists available at ScienceDirect The Journal of Foot & Ankle Surgery journal homepage: www.jfas.org Tips, Quips, and Pearls “Tips, Quips, and Pearls” is a special section in The Journal of Foot & Ankle Surgery which is devoted to the sharing of ideas to make the practice of foot and ankle surgery easier. We invite our readers to share ideas with us in the form of special tips regarding diagnostic or surgical procedures, new devices or modifications of devices for making a surgical procedure a little bit easier, or virtually any other “pearl” that the reader believes will assist the foot and ankle surgeon in providing better care. Please address your tips to: D. Scot Malay, DPM, MSCE, FACFAS, Editor, The Journal of Foot & Ankle Surgery, PO Box 590595, San Francisco, CA 94159-0595; E-mail: [email protected] Hallux Varus as Complication of Foot Compartment Syndrome Paul Dayton, DPM, MS, FACFAS 1, Jean Paul Haulard, DPM, MS 2 1 Director, Podiatric Surgical Residency, Trinity Regional Medical Center, Fort Dodge, IA 2 Resident, Trinity Regional Medical Center, Fort Dodge, IA article info abstract Keywords: Hallux varus can present as a congenital deformity or it can be acquired secondary to trauma, surgery, or deformity neuromuscular disease. In the present report, we describe the presence of hallux varus as a sequela of great toe calcaneal fracture with entrapment of the medial plantar nerve in the calcaneal tunnel and recommend that metatarsophalangeal joint clinicians be wary of this when they clinically, and radiographically, evaluate patients after calcaneal fracture. -

On the Position and Course of the Deep Plantar Arteries, with Special Reference to the So-Called Plantar Metatarsal Arteries
Okajimas Fol. anat. jap., 48: 295-322, 1971 On the Position and Course of the Deep Plantar Arteries, with Special Reference to the So-Called Plantar Metatarsal Arteries By Takuro Murakami Department of Anatomy, Okayama University Medical School, Okayama, Japan -Received for publication, June 7, 1971- Recently, we have confirmed that, as in the hand and foot of the monkey (Koch, 1939 ; Nishi, 1943), the arterial supply of the human deep metacarpus is composed of two layers ; the superficial layer on the palmar surfaces of the interosseous muscles and the deep layer within the muscles (Murakami, 1969). In that study, we pointed out that both layers can be classified into two kinds of arteries, one descending along the boundary of the interosseous muscles over the metacarpal bone (superficial and deep palmar metacarpal arteries), and the other de- scending along the boundary of the muscles in the intermetacarpal space (superficial and deep intermetacarpal arteries). In the human foot, on the other hand, the so-called plantar meta- tarsal arteries are occasionally found deep to the plantar surfaces of the interosseous muscles in addition to their usual positions on the plantar surfaces of the muscles (Pernkopf, 1943). And they are some- times described as lying in the intermetatarsal spaces (Baum, 1904), or sometimes descending along the metatarsal bones (Edwards, 1960). These circumstances suggest the existence in the human of deep planta of the two arterial layers and of the two kinds of descending arteries. There are, however, but few studies on the courses and positions of the deep plantar arteries, especially of the so-called plantar metatarsal arteries. -

Atlas of the Facial Nerve and Related Structures
Rhoton Yoshioka Atlas of the Facial Nerve Unique Atlas Opens Window and Related Structures Into Facial Nerve Anatomy… Atlas of the Facial Nerve and Related Structures and Related Nerve Facial of the Atlas “His meticulous methods of anatomical dissection and microsurgical techniques helped transform the primitive specialty of neurosurgery into the magnificent surgical discipline that it is today.”— Nobutaka Yoshioka American Association of Neurological Surgeons. Albert L. Rhoton, Jr. Nobutaka Yoshioka, MD, PhD and Albert L. Rhoton, Jr., MD have created an anatomical atlas of astounding precision. An unparalleled teaching tool, this atlas opens a unique window into the anatomical intricacies of complex facial nerves and related structures. An internationally renowned author, educator, brain anatomist, and neurosurgeon, Dr. Rhoton is regarded by colleagues as one of the fathers of modern microscopic neurosurgery. Dr. Yoshioka, an esteemed craniofacial reconstructive surgeon in Japan, mastered this precise dissection technique while undertaking a fellowship at Dr. Rhoton’s microanatomy lab, writing in the preface that within such precision images lies potential for surgical innovation. Special Features • Exquisite color photographs, prepared from carefully dissected latex injected cadavers, reveal anatomy layer by layer with remarkable detail and clarity • An added highlight, 3-D versions of these extraordinary images, are available online in the Thieme MediaCenter • Major sections include intracranial region and skull, upper facial and midfacial region, and lower facial and posterolateral neck region Organized by region, each layered dissection elucidates specific nerves and structures with pinpoint accuracy, providing the clinician with in-depth anatomical insights. Precise clinical explanations accompany each photograph. In tandem, the images and text provide an excellent foundation for understanding the nerves and structures impacted by neurosurgical-related pathologies as well as other conditions and injuries. -

Appendix B: Muscles of the Speech Production Mechanism
Appendix B: Muscles of the Speech Production Mechanism I. MUSCLES OF RESPIRATION A. MUSCLES OF INHALATION (muscles that enlarge the thoracic cavity) 1. Diaphragm Attachments: The diaphragm originates in a number of places: the lower tip of the sternum; the first 3 or 4 lumbar vertebrae and the lower borders and inner surfaces of the cartilages of ribs 7 - 12. All fibers insert into a central tendon (aponeurosis of the diaphragm). Function: Contraction of the diaphragm draws the central tendon down and forward, which enlarges the thoracic cavity vertically. It can also elevate to some extent the lower ribs. The diaphragm separates the thoracic and the abdominal cavities. 2. External Intercostals Attachments: The external intercostals run from the lip on the lower border of each rib inferiorly and medially to the upper border of the rib immediately below. Function: These muscles may have several functions. They serve to strengthen the thoracic wall so that it doesn't bulge between the ribs. They provide a checking action to counteract relaxation pressure. Because of the direction of attachment of their fibers, the external intercostals can raise the thoracic cage for inhalation. 3. Pectoralis Major Attachments: This muscle attaches on the anterior surface of the medial half of the clavicle, the sternum and costal cartilages 1-6 or 7. All fibers come together and insert at the greater tubercle of the humerus. Function: Pectoralis major is primarily an abductor of the arm. It can, however, serve as a supplemental (or compensatory) muscle of inhalation, raising the rib cage and sternum. (In other words, breathing by raising and lowering the arms!) It is mentioned here chiefly because it is encountered in the dissection.