Medical Product Quality Report – COVID-19 Issues
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Community-Led Participatory Budgeting in Bangalore: Learning from Successful Cases
Community-led Participatory Budgeting in Bangalore: Learning from Successful Cases by ELIZABETH M. CLAY Bachelor of Arts, Urban Studies, 2002 Columbia University Submitted to the Department of Urban Studies and Planning in partial fulfillment of the requirements for the degree of MASTER IN CITY PLANNING at the Massachusetts Institute of Technology June 2007 @2007 Elizabeth Clay All rights reserved. The author hereby grants to MIT permission to reproduce and distribute publicly paper and electronic copies of this thesis document in whole or in part. Signature of Author: epartment of Urban Studies and Planning May 24, 2007 Certified -- ---- ~r ~· ,,,, - ,, S- - Ceasar McDowell Professor of the Practice of Community Development, MIT Department of Urban Studies and Planning Thesis Supervisor Accepted by: Langley Keyes Chairman, Master in City Planning Committee Department of Urban Studies and Planning I MASSACHU$ETTS INSTITUTE MASSACHUSETTS INSTITUTE OF TECHNOLOGY JUL 18 2007 LIBRARIES Community-led Participatory Budgeting in Bangalore: Learning from Successful Cases by ELIZABETH M. CLAY Submitted to the Department of Urban Studies and Planning on May 24, 2007 in Partial Fulfillment of the Requirements for the Degree of Master in City Planning at the Massachusetts Institute of Technology ABSTRACT Urban India is rapidly growing, and in cities like Bangalore, the dramatic changes have both positive and negative impacts. Citizens express concern about the capacity and credibility of local government and corporate sector in leading local development. In contrast to rural India where the 73rd amendment helped spur citizen participation in local decision-making, in urban India there have been limited channels for citizens to participate in governance outside of the electoral process. -

Predictive QSAR Tools to Aid in Early Process Development of Monoclonal Antibodies
Predictive QSAR tools to aid in early process development of monoclonal antibodies John Micael Andreas Karlberg Published work submitted to Newcastle University for the degree of Doctor of Philosophy in the School of Engineering November 2019 Abstract Monoclonal antibodies (mAbs) have become one of the fastest growing markets for diagnostic and therapeutic treatments over the last 30 years with a global sales revenue around $89 billion reported in 2017. A popular framework widely used in pharmaceutical industries for designing manufacturing processes for mAbs is Quality by Design (QbD) due to providing a structured and systematic approach in investigation and screening process parameters that might influence the product quality. However, due to the large number of product quality attributes (CQAs) and process parameters that exist in an mAb process platform, extensive investigation is needed to characterise their impact on the product quality which makes the process development costly and time consuming. There is thus an urgent need for methods and tools that can be used for early risk-based selection of critical product properties and process factors to reduce the number of potential factors that have to be investigated, thereby aiding in speeding up the process development and reduce costs. In this study, a framework for predictive model development based on Quantitative Structure- Activity Relationship (QSAR) modelling was developed to link structural features and properties of mAbs to Hydrophobic Interaction Chromatography (HIC) retention times and expressed mAb yield from HEK cells. Model development was based on a structured approach for incremental model refinement and evaluation that aided in increasing model performance until becoming acceptable in accordance to the OECD guidelines for QSAR models. -

Comparative Safety and Efficacy of Anti-PD-1 Monotherapy, Chemotherapy
Lv et al. Journal for ImmunoTherapy of Cancer (2019) 7:159 https://doi.org/10.1186/s40425-019-0636-7 COMMENTARY Open Access Comparative safety and efficacy of anti-PD- 1 monotherapy, chemotherapy alone, and their combination therapy in advanced nasopharyngeal carcinoma: findings from recent advances in landmark trials Jia-Wei Lv1†, Jun-Yan Li1†, Lin-Na Luo2†, Zi-Xian Wang2* and Yu-Pei Chen1* Abstract Recent phase 1–2 trials reported manageable safety profiles and promising antitumor activities of anti-PD-1 drugs (pembrolizumab, nivolumab, camrelizumab and JS001) with/without chemotherapy in recurrent/metastatic nasopharyngeal carcinoma (RM-NPC), however head-to-head comparison among these regimens is lacking. We aimed to comprehensively compare the efficacy and safety of different anti-PD-1 drugs, standard chemotherapy, and their combination therapy in RM-NPC. Adverse event (AE) and objective response rate (ORR) were assessed. The pooled incidence rates of grade 1–5/3–5 AEs were 74.1%/29.6, 54.2%/17.4, 92.3%/24.5, 96.8%/16.1, 91.2%/42.8, and 100%/87.9% for pembrolizumab, nivolumab, JS001, camrelizumab, chemotherapy and camrelizumab+chemotherapy, respectively, which suggested that nivolumab and pembrolizumab exhibited the optimal safety regarding grade 1–5 AEs whereas camrelizumab and nivolumab regarding grade 3–5 AEs. As second- or later-line therapy, ORR was higher with camrelizumab (34.1%), followed by pembrolizumab (26.3%), JS001 (23.3%), and nivolumab (19.0%); whereas ORR with first-line nivolumab reached 40%. Additionally, first-line camrelizumab+chemotherapy achieved a dramatically higher ORR than that with chemotherapy alone (90.9% vs. -

Download This PDF File
Med J Chin PLA, Vol. 42, No. 12, December 1, 2017 ǂ1029 䃲eڝeᠳࢃ̺ ӵϔߙᡇϊҍᣴ໓͌ܠᣴ̹ࢴҝᣱ ͙ࡧጴࡻцᕑ䃶ܲц ᕑࡧ႒̿͆ༀцۇ͙Ϧℽ㼏ᩪ 䛹⫳ࡧ႒̿͆ༀцۇ͙Ϧℽ㼏ᩪ ͙ࡧጴࡻцᕑ䃶ܲцᕑ䃶โ̿͆ༀц 喞ᕑٷ䩚䃹]Ȟ݇ѐ喞㵬ᕓнڟ] [͙పܲㆧण]ȞR605.97ȞȞȞȞ[᪳⡚ᴳᔃⴭ]ȞAȞȞȞȞ[᪳「㑂ण]Ȟ0577-7402(2017)12-1029-10 [DOI]Ȟ10.11855/j.issn.0577-7402.2017.12.02 Chinese emergency medicine expert consensus on diagnosis and treatment of traumatic hemorrhagic shock Emergency Medicine Branch of Chinese Medical Doctor Association People’s Liberation Army Professional Committee of Emergency Medicine People’s Liberation Army Professional Committee of Critical Care Medicine Professional Committee of Emergency Surgery, Emergency Medicine Branch of Chinese Medical Doctor Association 1ȞẮȞȞ䔜 ㏒10%⤯ڔѐ᭛ᠳᱦᷜ߇҈⩔κϦѿऺᝬ䕌⮰ᱦѿ㏿ᲰႸ᪠ᕓ⮰ⵠ౻সߋ㘩䯈ⶹȠᢚWHO㐋䃍喏݇ 40ᆭБ̷Ϧ㓐⮰仂㺭₧ఌ[1]Ƞ⤯ڔ⮰₧ύস16%⮰㜠₷⫱ҷఌ݇ѐᝬ㜠喏सᬢ݇ѐ᭛ ᄽȟ㏰㏳╸∔̹䋟ȟ㏲㘊Џ䅎㈶Νসۻ᭛ᠳ݇ѐ䕌ᱦѿ๓䛻㵬ᝬ㜠ᰵᩴᓖ⣛㵬䛻ٷѐ㵬ᕓн݇ ፤፤ऴᎢѺ㵬ࢷ(͵ͦᩢ㑕ࢷ┯90mmHg喏㘵ࢷ┯20mmHg喏ᝂ࣋ᰵ倄㵬ٷஔჄߋ㘩ःᢋ⮰⫱⤲⩋⤲䓳⼷Ƞн ࢷ㔱ᩢ㑕ࢷ㜖ദ㏫̷䭹Ĺ40mmHg)Ƞ30%~40%⮰݇ѐᗏ㔱₧ύ᭛ఌ㵬䓳ๆᝬ㜠喏ₐㆧᗏ㔱͙喏ᰵ̬䘔ܲ ఌͦ䩅䄛⮰⇧ᵴࣶ̹ᖜᑿ⮰⇧⫃ᣖ㔸₧ύ喏ࢌ10%~20%Ƞᕑᕓ㵬᭛݇ѐ仂㺭⮰छ䶰䭞ᕓ₧ఌ[2-3]Ƞ ᄽๆஔჄߋ㘩䯈ⶹ㐨ऴᒭۻ䛹㺭喏छᰵᩴڟᄥκ͑䛹݇ѐᗏ㔱㜟ٷ㵬喏㏌㵬ᕓнܦࣶᬢȟᔗ䕋ᣓݢ (multiple organ dysfunction syndrome喏MODS)⮰ࣽ⩋喏䭹Ѻ₧ύ⢳Ƞ ⮰ᕑ䃶ٷ䃲ᬔ㻰㠯স倄݇ѐ㵬ᕓнڝᠳࢃȠ᱘ڟᕑ⇧⮰Ⱔ㉓ٷⰚݹᅆᬌ݇ѐ㵬ᕓн ⇧喏ͦᕑ䃶ࡧጴӇ䃶⫃ӉᢚȠ ⤲⩋⤲⫱⮰ٷ2Ȟ݇ѐ㵬ᕓн ᭛㵬ქ䛻̺㵬ネქ⼛⮰̹ࡥ䙹喏䕌โঔ㏰㏳╸∔̹䋟喏Ϻ㔸ᑁٴ⮰⫱⤲⩋⤲ऄࡂ仂ٷѐ㵬ᕓн݇ 㘻ஔჄ⮰㐓ࣽᕓᢋჟȠڱ㵬䯈ⶹБࣶ܉䊣ᓚᓖ⣛ऄࡂȟ⅓Џ䅎ߔ߇႒ᐮ፤ȟ►⫳ࣹᏀȟ ᭛䛹㺭㘻ڢᰬᵥ᱘⮰⫱⤲⩋⤲ᩥऄ᭛㵬ᝬ㜠⮰ᓚᓖ⣛ߋ㘩䯈ⶹ喏ᅐٷ2.1Ȟᓚᓖ⣛ऄࡂȞ݇ѐ㵬ᕓн ၼὍᐻ(damage associatedܲڟϓ⩋ᢋѐⰤٷஔᓚᓖ⣛ᩥऄȠᄨ㜠ᓚᓖ⣛ߋ㘩䯈ⶹ⮰ͧ㺭ᱦݢ࠱᠘喝Ŗн Ꮐむࣶᣓᕓ►⫳ࣹᏀ喏ᑁ䊣㵬⫗ٹ㯷⮩স倄䓭⼧⢳㯷⮩1㼒ࣽٷmolecular patterns喏DAMP)[4-5]喏ຮ☙н 㵬㈧܉⯚ᢋѐᑁ䊣ڱᄽ喏ᰬ㏴ᄨ㜠㏰㏳╸∔̹䋟ȟ㏲㘊㑦⅓喞ŗۻ⯚ᢋѐȟℇ㏲㵬ネ⍃ȟᓖ⣛ქ䛻ڱネ 㐋⓬≧ȟᓚ㵬ᴿᒎ喏䭧ඊℇ㏲㵬ネࣶ㵬ネ㜾㑕ߋ㘩䯈ⶹ喏ߌ䛹㏰㏳㑦㵬㑦⅓喞Ř݇ѐᝬ㜠⮰ᠭ㐙ȟᑦ◴ ᓚᓖ⣛䯈ⶹȠޓߋ㘩喏ᄨ㜠ࣹᄰᕓ㵬ネ㜾㑕ߋ㘩㈶Ν喏ߌ⇸ܲڱ⮰ݦ⓬ᒝ৹⺊㏻ ⅓̺(ᗏ㔱ႄ⅓Џ䅎ߔ߇႒ᐮ፤喏࢟⅓ӇᏀ(DO2ٷ2.2Ȟ⅓Џߔ߇႒ᐮ፤ࣶ㏲㘊Џ䅎ᩥऄȞ݇ѐ㵬ᕓн -

Urinary Trypsin Inhibitor: Miraculous Medicine in Many Surgical Situations?
Korean J Anesthesiol 2010 Apr; 58(4): 325-327 Editorial DOI: 10.4097/kjae.2010.58.4.325 Urinary trypsin inhibitor: miraculous medicine in many surgical situations? Jong In Han Department of Anesthesiology and Pain Medicine, School of Medicine, Ewha Womans University, Seoul, Korea Recently, we encounter several articles regarding urinary Trypsin inhibitors act to suppress the proteolytic action trypsin inhibitor (UTI) published nationally [1,2]. When we take of trypsin on a variety of tissues and exert a localized anti- a glance at these articles, it feels like UTI acts as a miraculous inflammatory effect [8]. Therefore UTI is indicated for acute medicine on patients under general anesthesia because of inflammatory disorders, including acute pancreatitis, systemic its protection effect against surgical stress. Yet, even after the inflammatory reaction syndrome, circulatory insufficiency, first report on antitryptic action of urine by Bauer and Reich Stevens-Johnson syndrome, Toxic epidermal necrolysis (TEN), III in 1909 [3]; the start of use of the term UTI by Astrup and disseminated intravascular coagulation (DIC) and multiple Sterndorff in 1955 [4]; and numerous animal experiments and organ failure [9]. Previous studies of UTI have focused mainly clinical research done about UTI (803 articles about UTI and on modulating inflammatory reaction. UTI attenuates the 982 articles about ulinastatin in SCOPUS), UTI is not yet to elevation of neutrophil elastase release, thereby blunting the be used commonly. Therefore, it is important to understand rise of pro-inflammatory cytokine level; however, the actual the reason behind this situation. According to the webpage of mechanism in vivo is not clear [10]. -

PRODUCT INFORMATION Dapansutrile Item No
PRODUCT INFORMATION Dapansutrile Item No. 24671 CAS Registry No.: 54863-37-5 Formal Name: 3-(methylsulfonyl)-propanenitrile Synonym: 3-methanesulfonyl Propanenitrile OO MF: C H NO S 4 7 2 S FW: 133.2 CN Purity: ≥98% Supplied as: A crystalline solid Storage: -20°C Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Dapansutrile is supplied as a crystalline solid. A stock solution may be made by dissolving the dapansutrilein the solvent of choice. Dapansutrile is soluble in the organic solvent DMSO, which should be purged with an inert gas at a concentration of approximately 2 mg/ml. Further dilutions of the stock solution into aqueous buffers or isotonic saline should be made prior to performing biological experiments. Ensure that the residual amount of organic solvent is insignificant, since organic solvents may have physiological effects at low concentrations. Organic solvent-free aqueous solutions of dapansutrile can be prepared by directly dissolving the crystalline solid in aqueous buffers. The solubility of dapansutrile in PBS, pH 7.2, is approximately 3 mg/ml. We do not recommend storing the aqueous solution for more than one day. Description Dapansutrile is a β-sulfonyl nitrile inhibitor of the NLRP3 inflammasome that inhibits the release of IL-1β and decreases caspase-1 levels in LPS-stimulated J774A.1 murine macrophages, human monocyte derived macrophages (HMDMs), and primary human blood neutrophils without affecting TNF-α release.1 It is selective for NLRP3 over NLRP4 and AIM2 inflammasomes at concentrations up to 100 μM. -

ATP-Binding and Hydrolysis in Inflammasome Activation
molecules Review ATP-Binding and Hydrolysis in Inflammasome Activation Christina F. Sandall, Bjoern K. Ziehr and Justin A. MacDonald * Department of Biochemistry & Molecular Biology, Cumming School of Medicine, University of Calgary, 3280 Hospital Drive NW, Calgary, AB T2N 4Z6, Canada; [email protected] (C.F.S.); [email protected] (B.K.Z.) * Correspondence: [email protected]; Tel.: +1-403-210-8433 Academic Editor: Massimo Bertinaria Received: 15 September 2020; Accepted: 3 October 2020; Published: 7 October 2020 Abstract: The prototypical model for NOD-like receptor (NLR) inflammasome assembly includes nucleotide-dependent activation of the NLR downstream of pathogen- or danger-associated molecular pattern (PAMP or DAMP) recognition, followed by nucleation of hetero-oligomeric platforms that lie upstream of inflammatory responses associated with innate immunity. As members of the STAND ATPases, the NLRs are generally thought to share a similar model of ATP-dependent activation and effect. However, recent observations have challenged this paradigm to reveal novel and complex biochemical processes to discern NLRs from other STAND proteins. In this review, we highlight past findings that identify the regulatory importance of conserved ATP-binding and hydrolysis motifs within the nucleotide-binding NACHT domain of NLRs and explore recent breakthroughs that generate connections between NLR protein structure and function. Indeed, newly deposited NLR structures for NLRC4 and NLRP3 have provided unique perspectives on the ATP-dependency of inflammasome activation. Novel molecular dynamic simulations of NLRP3 examined the active site of ADP- and ATP-bound models. The findings support distinctions in nucleotide-binding domain topology with occupancy of ATP or ADP that are in turn disseminated on to the global protein structure. -

A Study on Ulinastatin in Preventing Post ERCP Pancreatitis
International Journal of Advances in Medicine Vedamanickam R et al. Int J Adv Med. 2017 Dec;4(6):1528-1531 http://www.ijmedicine.com pISSN 2349-3925 | eISSN 2349-3933 DOI: http://dx.doi.org/10.18203/2349-3933.ijam20175083 Original Research Article A study on ulinastatin in preventing post ERCP pancreatitis R. Vedamanickam1, Vinoth Kumar2*, Hariprasad2 1Department of Medicine, 2Department of Gasto and Hepatology , SREE Balaji Medical College and Hospital, Chrompet, Chennai, Tamil Nadu, India Received: 19 September 2017 Accepted: 25 October 2017 *Correspondence: Dr. Vinothkumar, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Pancreatitis remains the major complication of endoscopic retrograde cholangiopancreatography (ERCP), and hyperenzymemia after ERCP is common. Ulinastatin, a protease inhibitor, has proved effective in the treatment of acute pancreatitis. The aim of this study was to assess the efficacy of ulinastatin, compare to placebo study to assess the incidence of complication due to ERCPP procedure. Methods: In this study a randomized placebo controlled trial, patients undergoing the first ERCP was randomizing to receive ulinastatin one lakh units (or) placebo by intravenous infusion one hour before ERCP for ten minutes duration. Clinical evaluation, serum amylase, ware analysed before the procedure 4 hours and 24 hours after the procedure. Results: Total of 46 patients were enrolled (23 in ulinastatin and 23 in placebo group). -

Modulation of the Inflammatory Response After Spinal Cord Injury
ADVERTIMENT. Lʼaccés als continguts dʼaquesta tesi queda condicionat a lʼacceptació de les condicions dʼús establertes per la següent llicència Creative Commons: http://cat.creativecommons.org/?page_id=184 ADVERTENCIA. El acceso a los contenidos de esta tesis queda condicionado a la aceptación de las condiciones de uso establecidas por la siguiente licencia Creative Commons: http://es.creativecommons.org/blog/licencias/ WARNING. The access to the contents of this doctoral thesis it is limited to the acceptance of the use conditions set by the following Creative Commons license: https://creativecommons.org/licenses/?lang=en MODULATION OF THE INFLAMMATORY RESPONSE AFTER SPINAL CORD INJURY Presented by Jesús Amo Aparicio ACADEMIC DISSERTATION To obtain the degree of PhD in Neuroscience by the Universitat Autònoma de Barcelona 2019 Directed by Dr. Rubèn López Vales Tutorized by Dr. Xavier Navarro Acebes INDEX SUMMARY Page 7 INTRODUCTION Page 13 - Spinal cord Page 15 - Spinal cord injury Page 17 - Incidence and causes Page 18 - Types of SCI Page 18 - Biological events after SCI Page 20 - Studying SCI Page 24 - Animal models Page 24 - Lesion models Page 24 - Current therapies for SCI Page 25 - Basic principles of the immune system Page 27 - Innate immune response Page 27 - Adaptive immune response Page 28 - Inflammatory response Page 29 - Inflammatory response after SCI Page 30 - Modulation of injury environment Page 36 - Interleukin 1 Page 36 - Interleukin 37 Page 40 - Interleukin 13 Page 44 OBJECTIVES Page 47 MATERIALS AND METHODS Page 51 -

Looking for Therapeutic Antibodies in Next Generation Sequencing Repositories
bioRxiv preprint doi: https://doi.org/10.1101/572958; this version posted March 10, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Title: Looking for Therapeutic Antibodies in Next Generation Sequencing Repositories. Authors: Konrad Krawczyk1*, Matthew Raybould2, Aleksandr Kovaltsuk2, Charlotte M. Deane2 1 NaturalAntibody, Hamburg, Germany 2 Oxford University Department of Statistics, Oxford, UK *Correspondence to [email protected] Abstract: Recently it has become possible to query the great diversity of natural antibody repertoires using Next Generation Sequencing (NGS). These methods are capable of producing millions of sequences in a single experiment. Here we compare Clinical Stage Therapeutic antibodies to the ~1b sequences from 60 independent sequencing studies in the Observed Antibody Space Database. Of the 242 post Phase I antibodies, we find 16 with sequence identity matches of 95% or better for both heavy and light chains. There are also 54 perfect matches to therapeutic CDR-H3 regions in the NGS outputs, suggesting a nontrivial amount of convergence between naturally observed sequences and those developed artificially. This has potential implications for both the discovery of antibody therapeutics and the legal protection of commercial antibodies. Introduction Antibodies are proteins in jawed vertebrates that recognize noxious molecules (antigens) for elimination. An organism expresses millions of diverse antibodies to increase the chances that some of them will be able to bind the foreign antigen, initiating the adaptive immune response. -
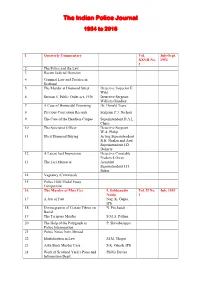
Index of the Indian Police Journal Issues from the Year 1954 to 2016
The Indian Police Journal 1954 to 2016 1 Quarterly Commentary Vol. July-Sept. XXVII No. 1954 3 2 The Police and the Law 3 Recent Judicial Decision 4 Criminal Law and Practice in Scotland 5 The Murder at Diamond Street Detective Inspector E. Wild 6 Section 5, Public Order act, 1936 Detective Sergeant William Grindley 7 A Case of Homicidal Poisoning Dr. Donald Teare 8 Previous Conviction Records Sergeant P.J. Nichols 9 The Case of the Headless Corpse Superintendent D.A.L. Chase 10 The Specialist Officer Detective Sergeant W.A. Philip 11 Illicit Diamond Buying Acting Superintendent B.H. Nealan and Asst. Superintendent J.D. Doherty 12 A Latent heel Impression Detective Constable Frederick Owen 13 The Lari Massacre Assistant Superintendent J.H. Baker 14 Vagrancy (Continued) 15 Police Gold Medal Essay Competition 16 The Murder of Miss Cox I. Sobhanadri Vol. II No. July 1955 Naidu 1 17 A Jest of Fate Nag. K. Gupta, IPS 18 Disintegration of Certain Fibres on N. Pitchandi Burial 19 The Tarapore Murder S.M.A. Pathan 20 The Help of the Polygraph in P. Shivabasappa Police Interrogation 21 Police Notes from Abroad 22 Identification in Law M.M. Thapar 23 Aska Bank Murder Case S.K. Ghosh, IPS 24 Work of Scotland Yard‘s Press and Phillis Davies Information Deptt. 25 Murder or Accident L. Forstner 26 The Finger Prints of Bahadur Khan Shiam Narain 27 A Chain of Forensic V.R. Kher, I.P. Vol. II No. January Laboratories in India 3 1956 28 Belbad Colliery Dacoity N.S. -

W W W .Bio Visio N .Co M
Biosimilar Monoclonal Antibodies Human IgG based monoclonal antibodies (mAbs) are the fastest-growing category of therapeutics for cancer therapy. Several mechanisms of tumor cell killing by antibodies (mAbs) can be summarized as: direct action through receptor blockade or induction of apoptosis; immune-mediated cell killing by complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC) or regulation of T cell function. Several monoclonal antibodies have received FDA approval for the treatment of a variety of solid tumors and hematological malignancies. BioVision is pleased to offer research grade biosimilars in human IgG format for your research needs. Our monoclonal antibodies are manufactured using recombinant technology with variable regions from the therapeutic antibody to achieve similar safety and efficacy. These antibodies can be used as controls for preclinical lead identification and potency assays for the development of novel therapeutics. Antibody Name Cat. No. Trade Name Isotype Size Anti-alpha 5 beta 1 Integrin (Volociximab), Human IgG4 Ab A1092 - IgG4 200 µg Anti-Beta-galactosidase, Human IgG1 Ab A1104 - IgG1 200 µg Anti-C5 (Eculizumab), Humanized Ab A2138 - IgG2/4 100 μg Anti-Carcinoembryonic antigen (Arcitumomab), Human IgG1 Ab A1096 - IgG1 200 µg Anti-CCR4 (Mogamulizumab), Human IgG1, kappa Ab A2005 - IgG1 200 μg Anti-CD11a (Efalizumab), Human IgG1 Ab A1089 Raptiva IgG1 200 µg Anti-CD20 (Rituximab), Chimeric Ab A1049 Mabthera IgG1 100 µg Anti-CD22 (Epratuzumab), Human IgG1 Ab A1445 LymphoCide IgG1 200 µg Anti-CD3 epsilon (Muromonab), Mouse IgG2a, kappa Ab A2008 - IgG2a 200 μg Anti-CD33 (Gemtuzumab), Human IgG4 Ab A1443 Mylotarg IgG4 200 µg Anti-CD38 (Daratumumab), Human IgG1 Ab A2151 Darzalex IgG1 100 μg www.biovision.com 155 S.