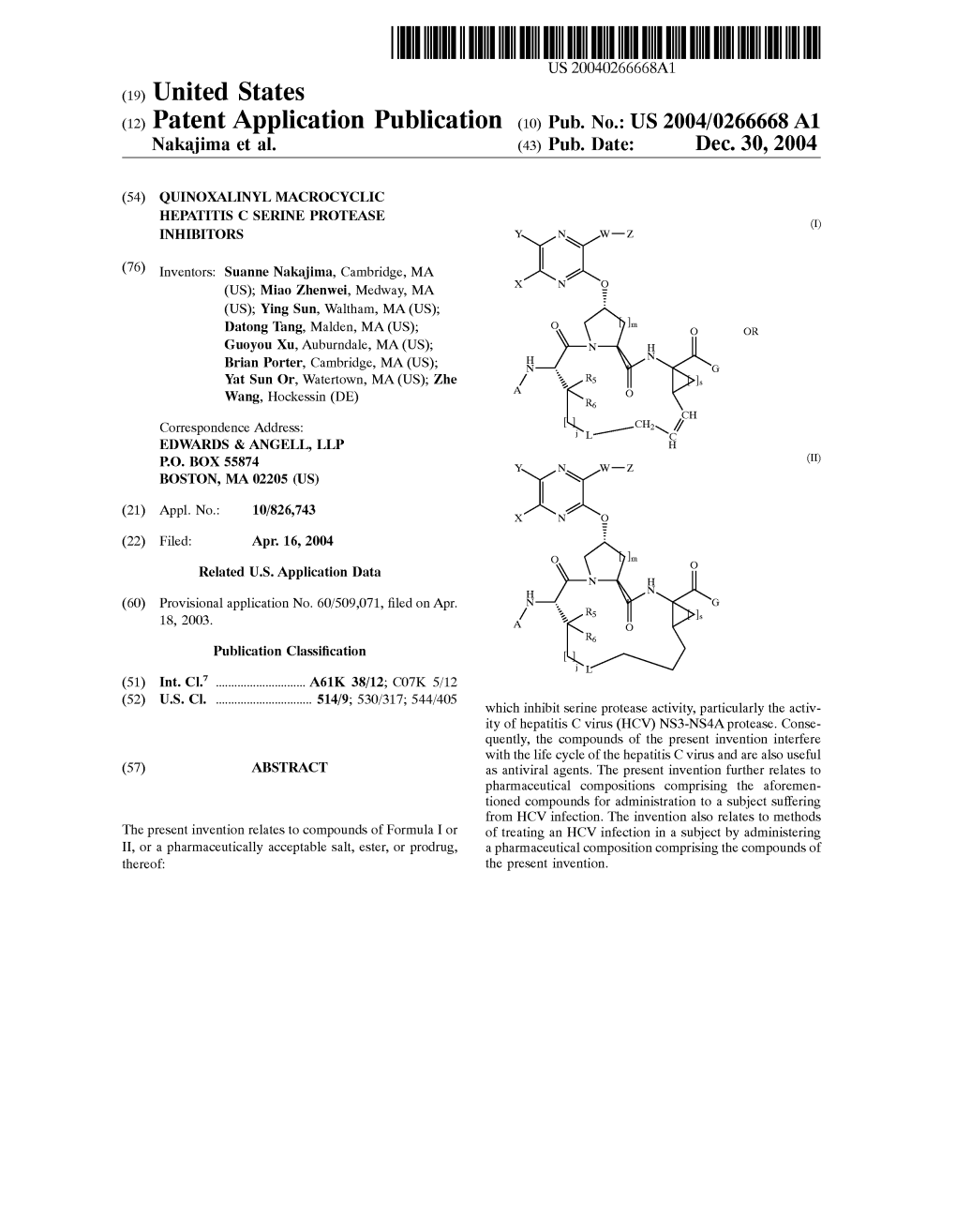
(12) Patent Application Publication (10) Pub. No.: US 2004/0266668A1 Nakajima Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1, 1, 1, 3, 3-Pentafluoropropane (HFC-245Fa) (CAS No. 460-73-1)
1, 1, 1, 3, 3-Pentafluoropropane (HFC-245fa) (CAS No. 460-73-1) JACC Report No. 44 Brussels, June 2004 1, 1, 1, 3, 3-Pentafluoropropane (HFC-245fa) ECETOC JACC REPORT No. 44 © Copyright - ECETOC European Centre for Ecotoxicology and Toxicology of Chemicals 4 Avenue E. Van Nieuwenhuyse (Bte 6), B-1160 Brussels, Belgium. All rights reserved. No part of this publication may be reproduced, copied, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the copyright holder. Applications to reproduce, store, copy or translate should be made to the Secretary General. ECETOC welcomes such applications. Reference to the document, its title and summary may be copied or abstracted in data retrieval systems without subsequent reference. The content of this document has been prepared and reviewed by experts on behalf of ECETOC with all possible care and from the available scientific information. It is provided for information only. ECETOC cannot accept any responsibility or liability and does not provide a warranty for any use or interpretation of the material contained in the publication. ECETOC JACC No. 44 1, 1, 1, 3, 3-Pentafluoropropane (HFC-245fa) 1,1,1,3,3-Pentafluoropropane (HFC-245fa) (CAS No. 460-73-1) CONTENTS EXECUTIVE SUMMARY 1 THE ECETOC SCHEME FOR THE JOINT ASSESSMENT OF COMMODITY CHEMICALS 2 1. SUMMARY AND CONCLUSIONS 3 2. IDENTITY, PHYSICAL, AND CHEMICAL PROPERTIES, ANALYTICAL METHODS 5 2.1 Identity 5 2.2 EC classification and labelling 5 2.3 Physical and chemical properties 5 2.4 Conversion factors 6 2.5 Analytical methods 7 2.5.1 In air 7 2.5.2 In water 7 3. -

Quantum Chemical Study of the Mechanism of the Catalytic
ISSN 00231584, Kinetics and Catalysis, 2013, Vol. 54, No. 2, pp. 157–167. © Pleiades Publishing, Ltd., 2013. Original Russian Text © D.E. Zavelev, G.M. Zhidomirov, R.A. Kozlovskii, 2013, published in Kinetika i Kataliz, 2013, Vol. 54, No. 2, pp. 166–176. Quantum Chemical Study of the Mechanism of the Catalytic Oxyethylation of Ethylene Glycol on PhosphorusDoped Titanium Dioxide: The Role of the Surface Phosphoryl and Hydroxyl Groups of the Catalyst D. E. Zaveleva,*, G. M. Zhidomirovb,c, and R. A. Kozlovskiid a Topchiev Institute of Petrochemical Synthesis, Russian Academy of Sciences, Moscow, 119991 Russia b Boreskov Institute of Catalysis, Siberian Branch, Russian Academy of Sciences, Novosibirsk, 630090 Russia c Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Moscow, 119991 Russia d Mendeleev University of Chemical Technology of Russia, Moscow, 125047 Russia *email: [email protected] Received June 25, 2012 Abstract—DFT calculations of the oxyethylation pathways of monoethylene glycol (MEG) and diethylene glycol (DEG) were performed on a model fragment of phosphorusdoped titanium dioxide (anatase). It was shown that the surface hydroxyl group of titanium dioxide, whose proton initiates C–O bond cleavage in the ethylene oxide molecule, plays the key role in the activation of the molecule. At the same time, the phospho ryl group –P(OH)2O activates the reactant molecule R (MEG, DEG, etc.) and carries out the synchronous proton transfer from R to the hydroxyl oxygen atom of titanium dioxide, thus restoring the catalyst structure and closing the catalytic cycle. This restructuring occurs synchronously in one step. The substitution of the catalyst hydroxyl groups by alkoxyl groups can influence oxyethylation occurring via the bimolecular nucleo philic substitution mechanism and can poison the catalyst in some cases. -

Novel Synthesis of Partially Fluorinated Ethers
Issue in Honor of Prof. Sukh Dev ARKIVOC 2003 (iii) 104-119 Alkylation of in situ generated fluorinated alkoxides: novel synthesis of partially fluorinated ethers G. K. Surya Prakash*, Jinbo Hu and George A. Olah Loker Hydrocarbon Research Institute and Department of Chemistry, University of Southern California, University Park, Los Angeles, CA 90089-1661 E-mail: [email protected] Dedicated to Professor Sukh Dev on the occasion of his 80th birthday (received 07 Jan 03; accepted 24 Feb 03; published on the web 27 Feb 03) Abstract Fluorinated ethers RfCF2OR were prepared from acyl chlorides RfCOCl, alkylating agents (such as dimethyl sulfate, alkyl triflates, alkyl mesylates and tosylates, etc.) and potassium fluoride in diglyme at 80~100 oC. The success and the efficiency of the reaction are affected by two factors: the stability and nucleophilicity of the fluorinated alkoxide generated in situ; and the other is the electrophilicity of the alkylating agents used. Keywords: Fluoroethers, fluoroacyl halides, fluoride ion, alkylation Introduction In general, carbon-fluorine bonds (C-F, ~116 kcal/mol) are stronger than carbon-hydrogen bonds (C-H, ~99 kcal/mol).1 However, not all organofluorine compounds are more stable than their non-fluorinated analogs, and sometimes they are even less stable. For instance, if a partially or fully fluorinated organic compounds contains an acidic hydrogen at the β position, HF elimination is observed. This is the main reason that α-fluoro alcohols are thermally unstable and 2 difficult to be synthesized or isolated. Formation of fluoromethanol (FCH2OH) from ethyl fluoroformate and from formyl fluoride was first reported by Olah and Pavlath3 in 1953, and Weinmayer4 in 1963 claimed the formation of fluoromethanol (25-30% in equilibrium) from a solution of paraformaldehyde in excess hydrogen fluoride. -

Fluoro-Silane As a Functional Monomer for Protein Conformational Imprinting
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 5-2011 Fluoro-Silane as a Functional Monomer for Protein Conformational Imprinting Yun Peng Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Biomedical Engineering and Bioengineering Commons Recommended Citation Peng, Yun, "Fluoro-Silane as a Functional Monomer for Protein Conformational Imprinting" (2011). All Graduate Theses and Dissertations. 906. https://digitalcommons.usu.edu/etd/906 This Dissertation is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. FLUORO-SILANE AS A FUNCTIONAL MONOMER FOR PROTEIN CONFORMATIONAL IMPRINTING by Yun Peng A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in Biological Engineering Approved: David W. Britt, Ph.D. Timothy E. Doyle, Ph.D. Major Professor Committee Member Marie K. Walsh, Ph.D. Soonjo Kwon, Ph.D. Committee Member Committee Member Jixun Zhan, Ph.D. Byron Burnham Committee Member Dean of Graduate Studies UTAH STATE UNIVERSITY Logan, Utah 2011 ii Copyright © Yun Peng 2011 All Rights Reserved iii ABSTRACT Fluoro-silane as a Functional Monomer for Protein Conformational Imprinting by Yun Peng, Doctor of Philosophy Utah State University, 2011 Major Professor: Dr. David W. Britt Department: Biological Engineering By using the technology of molecularly imprinted polymer (MIP), we propose to synthesize a protein conformational imprint that also acts as a plastic enzyme, inducing protein structural transitions. -

(12) United States Patent (10) Patent No.: US 8,501,891 B2 Nozue Et Al
US008501891B2 (12) United States Patent (10) Patent No.: US 8,501,891 B2 NOZue et al. (45) Date of Patent: Aug. 6, 2013 (54) ETHYLENE-o-OLEFIN COPOLYMER AND (56) References Cited MOLDED ARTICLE U.S. PATENT DOCUMENTS (75) Inventors: Yoshinobu Nozue, Ichihara (JP); 5,962,615 A 10/1999 Kojoh et al. Yasutoyo Kawashima, Ichihara (JP); 2002/0143124 A1 10/2002 Ogane 2003/0060579 A1 3/2003 Oshima et al. Naoko Ochi, Chiba (JP) 2006, OO89476 A1 4/2006 Iseki et al. 2006/O199925 A1 9/2006 Matsuura et al. (73) Assignee: Sumitomo Chemical Company, 2007/0093.627 A1 4/2007 Iseki et al. Limited, Tokyo (JP) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this CN 1765943. A 5, 2006 CN 1939.943. A 4/2007 patent is extended or adjusted under 35 EP O339571 A1 11, 1989 U.S.C. 154(b) by 423 days. JP 1-292009 A 11, 1989 JP O7-238114 A 9, 1995 (21) Appl. No.: 12/745,178 JP 08-0597O6 A 3, 1996 JP 2003-0961.25 A 4/2003 JP 2003-171412 A 6, 2003 (22) PCT Filed: Nov. 27, 2008 JP 2005-2333 A 1, 2005 JP 2005-206777 A 8, 2005 (86). PCT No.: PCT/UP2008/072000 JP 2005-24.8013. A 9, 2005 JP 2005-281541. A * 10, 2005 S371 (c)(1), JP 2006-233208 A 9, 2006 (2), (4) Date: Jul. 16, 2010 OTHER PUBLICATIONS (87) PCT Pub. No.: WO2009/069823 Mino et al., (JP 2005-281541) Oct. 13, 2005; abstract and translation in English.* PCT Pub. -

Reactive Chlorine Compounds in the Atmosphere
CHAPTER 1 Reactive Bromine Compounds O.N.Singh 1 · P.Fabian 2 1 Department of Applied Physics, Institute of Technology, Banaras Hindu University, Varanasi- 221 005, India. E-mail: [email protected] 2 University of Munich, Lehrstuhl für Bioklimatologie und Immissionsforschung, Am Hochanger 13, D-85354 Freising-Weihenstephan, Germany. E-mail: [email protected] Bromine, a minor constituent in the Earth’s atmosphere – with its 50-fold higher efficiency of ozone destruction compared to chlorine – contributes significantly to the ozone hole formation and wintertime stratospheric ozone depletion over northern mid and high latitudes.In addition ozone episodes observed in the Arctic during polar sunrise are solely due to atmospheric bromine.CH3Br, CH2Br2 and CHBr3 are the major brominated gases in the atmosphere, of which CH3Br being most abundant, contributes about 50% and CH2Br2 around 7 to 10% of the total organic stratospheric bromine.Bromocarbons with shorter lifetimes like CHBr3 ,CH2BrCl, CHBr2Cl, CHBrCl2 and CH2BrI decompose before reaching the stratosphere, and are responsible for the ozone episodes.But for 3CHBr, which has also significant anthropogenic sources, all the aforementioned bromocarbons are mostly of marine origin.Halons (H-1211, H-1301, H-2402, H-1202) are solely anthropogenic and are far more stable.They decompose only after reaching the stratosphere.It is estimated that 39% of the stratospheric organic bromine (ª 7 pptv) loading is due to these halons.Increa- ses are being still registered in the atmospheric abundance of halons in spite of production restrictions.Though extensively investigated,the existing knowledge with regard to the pro- duction and degradation of atmospheric bromine gases, is not commensurate with its importance. -

MALDI Matrix and MALDI Method
(19) & (11) EP 2 060 919 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 20.05.2009 Bulletin 2009/21 G01N 33/68 (2006.01) H01J 49/04 (2006.01) (21) Application number: 07120550.4 (22) Date of filing: 13.11.2007 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Wuijckhuijse, Arjan Laurens HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE 3331 GP Zwijndrecht (NL) SI SK TR • Kientz, Charles Eliza Designated Extension States: 2611 Gl Delft (NL) AL BA HR MK RS (74) Representative: Hatzmann, Martin (71) Applicant: Nederlandse Organisatie voor Vereenigde Toegepast- Johan de Wittlaan 7 Natuurwetenschappelijk Onderzoek TNO 2517 JR Den Haag (NL) 2628 VK Delft (NL) (54) MALDI matrix and MALDI method (57) The invention is directed to a matrix material for wherein X is chosen from S, O or N, and wherein R1 and MALDI mass spectrometry, to a matrix composition for R2 are independently chosen from hydrogen, methyl, MALDI mass spectrometry, in particular for aerosol MAL- methoxy, ethoxy, and propoxy, or wherein R1 and R2 are DI mass spectrometry, to a MALDI mass spectrometry taken together to form an optionally substituted aromatic method, in particular an aerosol MALDI mass spectrom- ring structure, optionally comprising one or more heter- etry method, to the use of a specific compound as a MAL- oatoms, DI matrix material, and to the use of a MALDI matrix com- or a tautomeric form thereof. position in a gas phase coating method. -

Controlling Transition Metal-Catalyzed Alkyne Annulations Utilizing Polarized Ynol Ethers
University of Denver Digital Commons @ DU Electronic Theses and Dissertations Graduate Studies 2020 Controlling Transition Metal-Catalyzed Alkyne Annulations Utilizing Polarized Ynol Ethers Brandon L. Coles-Taylor Follow this and additional works at: https://digitalcommons.du.edu/etd Part of the Organic Chemistry Commons Controlling Transition Metal-Catalyzed Alkyne Annulations Utilizing Polarized Ynol Ethers ______________ A Dissertation Presented to the Faculty of the College of Natural Sciences and Mathematics University of Denver ____________ In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy ____________ by Brandon L. Coles-Taylor June 2020 Advisor: Brian Michel, PhD ©Copyright by Brandon L. Coles-Taylor 2020 All Rights Reserved Author: Brandon L. Coles-Taylor Title: Controlling Transition Metal-Catalyzed Alkyne Annulations Utilizing Polarized Ynol Ethers Advisor: Brian Michel, PhD Degree Date: June 2020 Abstract Transition metal-catalyzed alkyne annulations have developed into incredibly powerful synthetic tools over there the past quarter century. These reactions provide rapid access to important organic scaffolds such as indole, quinoline, isoquinoline, indene, and isocoumarin scaffolds. Transition metal mediated alkyne annulations have proven invaluable in synthetic fields, such as natural product total synthesis, by offering efficient pathways to otherwise synthetically difficult to access substrates. Foundational works performed by chemist such as Larock, Ackermann, Satoh, and Miura have been established through relying upon the usage of symmetrical alkynes. When unsymmetrical alkynes are used in annulation processes mixtures of regioisomers are often isolated. While methodologies have been developed which regioselectively deliver annulation products, the regioselective nature of these reactions is often empirically determined and obtained with little synthetic design to impact the alkyne migratory insertion step of the catalytic cycle. -

Trifluoromethyl Ethers – Synthesis and Properties of an Unusual Substituent
Trifluoromethyl ethers – synthesis and properties of an unusual substituent Frédéric R. Leroux*1, Baptiste Manteau1, Jean-Pierre Vors2 and Sergiy Pazenok3 Review Open Access Address: Beilstein Journal of Organic Chemistry 2008, 4, No. 13. 1Laboratoire de Stéréochimie, Université Louis Pasteur (ECPM), doi:10.3762/bjoc.4.13 CNRS, 25 rue Becquerel, F – 67087 Strasbourg Cedex 2, France, 2Bayer CropScience SA, Centre de Recherches de La Dargoire, Received: 08 January 2007 14-20 Rue Pierre Baizet, F – 69009 Lyon, France and 3Bayer Accepted: 22 April 2008 CropScience AG, Alfred-Nobel-Strasse 50, D – 40789 Monheim, Published: 29 April 2008 Germany © 2008 Leroux et al; licensee Beilstein-Institut. Email: License and terms: see end of document. Frédéric R. Leroux* - [email protected] * Corresponding author Abstract After nitrogen, fluorine is probably the next most favorite hetero-atom for incorporation into small molecules in life science- oriented research. This review focuses on a particular fluorinated substituent, the trifluoromethoxy group, which is finding increased utility as a substituent in bioactives, but it is still perhaps the least well understood fluorine substituent in currency. The present review will give an overview of the synthesis, properties and reactivity of this important substituent. Introduction Nowadays, fluorine containing compounds are synthesized in Fluorine as a substituent in active ingredients plays a signi- pharmaceutical research on a routine basis and about 10% of all ficant and increasingly important role. Currently about 15% of marketed pharmaceuticals contain a fluorine atom. There has the pesticides listed in the 13th edition of the Pesticide Manual been an enormous increase in the use of fluorine containing contain at least one fluorine atom. -

Using JCP Format
Estimating Solid–Liquid Phase Change Enthalpies and Entropies Cite as: Journal of Physical and Chemical Reference Data 28, 1535 (1999); https:// doi.org/10.1063/1.556045 Submitted: 07 January 1999 . Published Online: 28 April 2000 James S. Chickos, William E. Acree, and Joel F. Liebman ARTICLES YOU MAY BE INTERESTED IN Phase Transition Enthalpy Measurements of Organic and Organometallic Compounds. Sublimation, Vaporization and Fusion Enthalpies From 1880 to 2010 Journal of Physical and Chemical Reference Data 39, 043101 (2010); https:// doi.org/10.1063/1.3309507 Heat Capacities and Entropies of Organic Compounds in the Condensed Phase. Volume III Journal of Physical and Chemical Reference Data 25, 1 (1996); https://doi.org/10.1063/1.555985 Enthalpies of Vaporization of Organic and Organometallic Compounds, 1880–2002 Journal of Physical and Chemical Reference Data 32, 519 (2003); https:// doi.org/10.1063/1.1529214 Journal of Physical and Chemical Reference Data 28, 1535 (1999); https://doi.org/10.1063/1.556045 28, 1535 © 1999 American Institute of Physics and American Chemical Society. Estimating Solid–Liquid Phase Change Enthalpies and Entropies James S. Chickosa… Department of Chemistry, University of Missouri–St. Louis, St. Louis Missouri 63121 William E. Acree, Jr. Department of Chemistry, University of North Texas, Denton, Texas 76203 Joel F. Liebman Department of Chemistry and Biochemistry, University of Maryland Baltimore County, Baltimore, Maryland 21250 Received January 7, 1999; revised manuscript received July 10, 1999 A group additivity method based on molecular structure is described that can be used ⌬Tfus ⌬Tfus to estimate solid–liquid total phase change entropy ( 0 Stpce) and enthalpy ( 0 Htpce) of organic molecules. -
(12) United States Patent (10) Patent No.: US 9,127,295 B2 Rondinone Et Al
US009 127295B2 (12) United States Patent (10) Patent No.: US 9,127,295 B2 Rondinone et al. (45) Date of Patent: Sep. 8, 2015 Vasconcelos, et al., 1998. Copper(II) Complexation Properties and (54) MICROBIAL-MEDIATED METHOD FOR Surfactant Activity of 3-N,N-Bis(2-hydroxyethyl)amino-2- METAL OXDENANOPARTICLE hydroxypropanesulfonic Acid and N-(2-Hydroxyethyl)piperazine FORMATION N9-2-hydroxypropanesulfonic Acid pH Buffers Which May Affect Trace Metal Speciation in in Vitro Studies. Analytical Biochemistry (75) Inventors: Adam J. Rondinone, Knoxville, TN 265, 193-201.* (US); Ji Won Moon, Oak Ridge, TN Limbach et al. Environ. Sci. Technol. 2008, 42,5828-5833.* Love L.J., et al. “Characterization of Bio-Synthesized Magnetic (US); Lonnie J. Love, Knoxville, TN Nanoparticles'. Proceedings of the 2005 IEEE/ASME International (US); Lucas W. Yeary, Painted Post, NY Conference on Advanced Intelligent Mechatronics, Monterey, Cali (US); Tommy J. Phelps, Knoxville, TN fornia (Jul 24-28, 2005). (US) Roh V. et al. “Isolation and Characterization of Metal-Reducing Thermoanaerobacter Strains from Deep Subsurface Environments of the Piceance Basin, Colorado”. Applied and Environmental (73) Assignee. UT-BATTELLE, LLC, Oak Ridge, TN Microbiology, 68(12): 6013-6020 (Dec. 2002). (US) Rondinone A.J. et al., “A Chemometric Approach for Predicting the Size of Magnetic Spinel Ferrite Nanoparticles from the Synthesis (*) Notice: Subject to any disclaimer, the term of this Conditions”. J. Phys. Chem. B., 104(33):7919-7922 (2000). patent is extended or adjusted under 35 Kieft T.L. et al., “Dissimilatory Reduction of Fe(III) and Other Elec U.S.C. 154(b) by 1184 days. -

Perfluorinated Dialkyl Peroxides
PERFLUORINATED DIALKYL PEROXIDES THEIR PROPERTIES AND SYNTHESES FROM PERFLUOROALKYL HYPOHALITES Inaugural-Dissertation to obtain the academic degree Doctor rerum naturalium submitted to the Department of Biology, Chemistry, Pharmacy of Freie Universität Berlin Jan Hendrick Nissen 2020 PERFLUORINATED DIALKYL PEROXIDES THEIR PROPERTIES AND SYNTHESES FROM PERFLUOROALKYL HYPOHALITES Inaugural-Dissertation to obtain the academic degree Doctor rerum naturalium submitted to the Department of Biology, Chemistry, Pharmacy of Freie Universität Berlin Jan Hendrick Nissen 2020 für meine Familie The work for the present dissertation has been conducted between November 2015 and February 2020 under the supervision of Prof. Dr. Sebastian Hasenstab-Riedel at the Institute of Chemistry and Biochemistry at the Department Biology, Chemistry and Pharmacy of Freie Universität Berlin. I certify that this thesis is entirely my own independent work. No other than the declared sources have been used, which are acknowledged as references. 1st referee: Prof. Dr. Sebastian Hasenstab-Riedel 2nd referee: Prof. Dr. Christian Müller Day of Submission: 27.02.2020 Day of Disputation: 03.07.2020 Acknowledgements Thank you Sebastian for giving me the opportunity to work on this attractive, challenging and diverse research project during my doctorate. Thank you very much for your constant support on my way to your working group here at Freie University of Berlin, your infectious passion for the subject, and constant willingness to help. Many thanks also go to Prof. Dr. Christian Müller for his interest in this work and the willing acceptance as the second referee. I am particularly grateful to Helmut Beckers, Simon Steinhauer, and Günther Thiele for the scientific exchange and the support that led to the success of this thesis.