Attached Leaves and Fruits of Myrtaceous Affinity from the Middle Eocene of Colorado
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Imported Parasitic Wasp Helps Control Red Gum Lerp Psyllid
UC Agriculture & Natural Resources California Agriculture Title Imported parasitic wasp helps control red gum lerp psyllid Permalink https://escholarship.org/uc/item/1f63j4hz Journal California Agriculture, 59(4) ISSN 0008-0845 Authors Dahlsten, Donald L. Daane, Kent M. Paine, Timothy D. et al. Publication Date 2005 Peer reviewed eScholarship.org Powered by the California Digital Library University of California RESEARCH ARTICLE ▼ Imported parasitic wasp helps control red gum lerp psyllid Donald L. Dahlsten Kent M. Daane Timothy D. Paine Karen R. Sime Andrew B. Lawson David L. Rowney William J. Roltsch John W. Andrews Jr. John N. Kabashima David A. Shaw Karen L. Robb James A. Downer* Pamela M. Geisel William E. Chaney Chuck A. Ingels The parasitoid Psyllaphaegus bliteus has Lucia G. Varela been released throughout California to Mary L. Bianchi control the red gum lerp psyllid, a pest of eucalyptus. Above, an adult P. bliteus uses Gary Taylor its ovipositor to place an egg inside the ▼ red gum lerp psyllid nymph. The parasitoid develops inside the psyllid nymph, which typically does not show any signs of parasit- years ago. Until recently, eucalyptus ism until the nymph reaches the fifth instar, The red gum lerp psyllid is an insect trees in California were relatively free when the parasitoid pupa — far left, white body, and left, dark body — can be seen native to Australia, where it feeds from damaging insect pests. Most of through the mummified psyllid. upon eucalyptus species. Since 1998 California’s native insects cannot feed on eucalyptus, which is well protected this psyllid has spread throughout Cal- from herbivores by chemicals such as ifornia, resulting in millions of dollars distasteful essential oils (which are fa- first found on river red gum in June in damage and control costs. -

Vegetation and Seed Bank of an Open-Scrub Bush Restinga Formation in the Southeastern Coast of Brazil
ISSN Printed: 0034-7744 ISSN digital: 2215-2075 Vegetation and seed bank of an open-scrub bush restinga formation in the Southeastern coast of Brazil Fernando Campanhã Bechara1, Lívia Zocatelli Salvador2, Raquel Almeida Ventura3, Larissa Regina Topanotti4*, Dionatan Gerber5, Izaclaudia Santana da Cruz6 & Marcelo Simonelli7 1. Universidade Tecnológica Federal do Paraná, Curso de Engenharia Florestal, Dois Vizinhos, Paraná, Brasil, University of Hawaii at Manoa; [email protected] 2. Faculdade de Saúde e Meio Ambiente (FAESA), Vitória, Espírito Santo, Brasil; [email protected] 3. Faculdade de Saúde e Meio Ambiente (FAESA), Vitória, Espírito Santo, Brasil; [email protected] 4. Universidade Federal de Santa Catarina, Divisão de Atividades Agropecuárias, Curitibanos, Santa Catarina, Brasil; [email protected] 5. Instituto Politécnico de Bragança, Programa de Pós-Graduação em Gestão de Recursos Florestais, Bragança, Bragança, Portugal; [email protected] 6. Instituto Federal de Educação, Ciência e Tecnologia Baiano, Valença, Bahia, Brasil; [email protected] 7. Instituto Federal de Educação, Ciência e Tecnologia do Espírito Santo, Vitória, Espírito Santo, Brasil; [email protected] * Correspondence Received 08-X-2019. Corrected 10-I-2020. Accepted 13-III-2020. ABSTRACT. Introduction: Restingas are coastal plain ecosystems located along Eastern Brazil, correspond- ing to about 5 000 km. The restinga vegetation is associated with the Atlantic rainforest biome and comprises four distinct main formation zones: coastal grasslands, shrublands, open-forests and marsh zones. Especially due to coastal urbanization, this is a threatened ecosystem that, through its different shrub formations, exhibits a unique mosaic as a result of the vegetation distribution in nuclei of different covering, physiognomy and floristic composition. -

United States Environmental Protection Agency Washington, D.C
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION MEMORANDUM DATE: March 1, 2013 SUBJECT: Crop Grouping – Part X: Analysis of the USDA IR-4 Petition to Amend the Crop Group Regulation 40 CFR § 180.41 (c) (25) and Commodity Definitions [40 CFR 180.1 (g)] Related to the Proposed Crop Group 23 Tropical and Subtropical Fruit – Edible Peel. PC Code: NA DP Barcode: NA Decision No.: NA Registration No.: NA Petition No.: NA Regulatory Action: Crop Grouping Regulation Risk Assessment Type: None Case No.: NA TXR No.: NA CAS No.: NA MRID No.: 482971-01 40 CFR: 180.41 (c) (25) and 180.1 (g) FROM: Bernard A. Schneider, Ph.D., Senior Plant Physiologist Chemistry and Exposure Branch Health Effects Division (7509P) THROUGH: Donna Davis and Donald Wilbur, Ph.D., Chairpersons HED Chemistry Science Advisory Council (ChemSAC) Health Effects Division (7509P) TO: Barbara Madden, Minor Use Officer Risk Integration, Minor Use, and Emergency Response Branch (RIMUERB) Registration Division (7505P) cc: IR-4 Project, Bill Barney, Jerry Baron, Dan Kunkel, Debbie Carpenter, Van Starner 2 ACTION REQUESTED: William P. Barney, Crop Grouping Project Coordinator, and Kathryn Homa, Assistant Coordinator, USDA Interregional Research Project No. 4 (IR-4), State Agricultural Experiment Station, Rutgers University has submitted a petition (November 16, 2010) on behalf of the IR-4 Project, and the Tropical Fruits Workgroup of the International Crop Grouping Consulting Committee (ICGCC) to establish a new Crop Group (40 CFR § 180.41) Crop Group 23, Tropical and Subtropical Fruit – Edible Peel Group, and propose addition of Commodity Definitions 40 CFR 180.1 (g). -

Eugenia Reinwardtiana (Blume) DC
Australian Tropical Rainforest Plants - Online edition Eugenia reinwardtiana (Blume) DC. Family: Myrtaceae Candolle, A.P. de (1828) Prodromus 3: 267. Common name: Cedar Bay Cherry; Beach Cherry; Cherry, Beach Stem Occasionally grows into a small tree seldom exceeding 30 cm dbh but also flowers and fruits as a shrub. Leaves Leaf blades about 2-9 x 1-5 cm, petioles about 0.1-0.6 cm long. Oil dots visible with a lens if not visible to the naked eye. Terminal buds and young shoots clothed in pale, prostrate, silky hairs. Flowers Inflorescence axillary, never truly terminal, bracts persistent, pubescent, present at anthesis, about 1.5 x 0.7 mm. Flower buds pubescent. Pedicel absent but peduncles long and slender and usually ending in one flower. Calyx tube (hypanthium) pubescent, 2-4 x 2-4 mm, calyx lobes rounded, Leaves and flower [not concave adaxially, more sparsely pubescent than the calyx tube (hypanthium), dimorphic, inner vouchered]. CC-BY J.L. Dowe lobes larger, about 2.5-3 mm long, +/- horizontal at anthesis. Petals +/- orbicular, glabrous except for the ciliate margins, about 3-3.5 mm diam., oil dots variable in number, about 30-70 per petal. Outer anther filaments about 3-5 mm long, anthers about 0.5-0.6 x 0.6-0.8 mm, gland inconspicuous, small, terminal, staminal disk broad, +/- level and conforming with the apex of the ovary. Ovules about 6-14 per locule. Style about 2.5-5.5 mm long, approximating the stamens. Fruit Fruits globular, depressed globular or ovoid, sometimes bilobed, attaining about 15-21 x 13-23 mm, calyx lobes persistent at the apex, about 2.5 mm long, pericarp succulent despite included fibres. -

Merrill (Myrtaceae) Leaves Collected from Goiás State, Brazil
Vol. 8(38), pp. 1134-1147, 10 October, 2014 DOI: 10.5897/JMPR2014.5395 Article Number: 8F92BC048382 ISSN 1996-0875 Journal of Medicinal Plant Research Copyright © 2014 Author(s) retain the copyright of this article http://www.academicjournals.org/JMPR Full Length Research Paper Structural organization and phytochemical analysis of Pimenta dioica (L.) Merrill (Myrtaceae) leaves collected from Goiás State, Brazil Leonardo Rodrigues Faria1, Rúbia Darc Machado1, Pedro Henrique Pimenta1, Leandra de Almeida Ribeiro Oliveira1, Josana de Castro Peixoto1, José Realino de Paula2, Pedro Henrique Ferri3 and Joelma Abadia Marciano de Paula1* 1Universidade Estadual de Goiás, Unidade de Ciências Exatas e Tecnológicas, Anápolis, GO, Brazil. 2Universidade Federal de Goiás, Laboratório de Pesquisa de Produtos Naturais, Goiânia, GO, Brazil. 3Universidade Federal de Goiás, Instituto de Química, Goiânia, GO, Brazil. Received 14 February, 2014; Accepted 9 October, 2014 Several biological properties are attributed to the Pimenta dioica plants that are native to Central America. Given the possible variabilities in both the morpho-anatomical and chemical aspects of the species that grow in different locations, this study aimed to contribute to the pharmacognostic study of P. dioica leaves from Brazil. Therefore, we morpho-anatomically described the plants, performed a phytochemical screening, analyzed the chemical composition of the essential oil and determined the quality parameters of P. dioica leaves that were collected in Goiás State. The leaves are simple and exhibit a camptodromous-brochidodromous venation pattern. The blades exhibit anomocytic stomata on the abaxial side, a dorsiventral mesophyll, secretory cavities on both sides, and a uniseriate epidermis. Trichomes can be observed in the petiole. -

Pimenta Dioica
Pimenta dioica • Caryophyllus pimento Mill. • Eugenia divaricata var. ovalis O.Berg • Eugenia micrantha (Kunth) DC. • Eugenia micrantha Bertol., Fl. Guatimal., 22. 1840. • Eugenia pimenta (L.) DC. • Eugenia pimenta var. longifolia (Sims) DC. • Eugenia pimenta var. ovalifolia DC. • Evanesca crassifolia Raf. • Evanesca micrantha Bertol. • Myrtus aromatica Salisb., Prodr. Stirp. Chap. Allerton 354. 1796. • Myrtus pimenta L., Sp. Pl., 472. 1753. • Myrtus pimenta Ortega • Myrtus pimenta var. brevifolia Hayne Pimenta dioica • Myrtus pimenta var. longifolia Sims • Myrtus piperita Sessé & Moc. 1 Taxonavigation • Pimenta aromatica Kostel. Familia: Myrtaceae • Pimenta officinalis Lindl., Collect. Bot., sub t. 19. Subfamilia: Myrtoideae 1821. Tribus: Myrteae Genus: Pimenta • Pimenta officinalis O.Berg Species: Pimenta dioica • Pimenta officinalis var. longifolia (Sims) O.Berg 2 Name • Pimenta officinalis var. ovalifolia (DC.) O.Berg • Pimenta officinalis var. tenuifolia O. Berg Pimenta dioica (L.) Merr., Contr. Gray Herb. 165:37. 1947. • Pimenta pimenta (L.) Cockerell • Pimenta pimenta (L.) H. Karst., Deut. Fl., 790. 2.1 Synonyms 1882, nom. inadmiss. Basionym • Pimenta vulgaris Lindl. • Pimentus aromatica (Salisb.) Raf. Sylva Tellur. • Myrtus dioica L., Syst. nat. ed. vol. 10, 1056. 1759. 158. 1838. Heterotypic • Pimentus vera Raf. 1 2 4 VERNACULAR NAMES 3 References • Contributions from the Gray Herbarium of Harvard University. Cambridge, MA 165:37. 1947 • USDA, ARS, Germplasm Resources Information Network. Pimenta dioica in the Germplasm Re- sources Information -
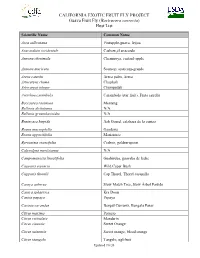
Guava Fruit Fly (Bactrocera Correcta) Host List
- cdfa USDA ------ ~--- CALIFORNIA EXOTIC FRUIT FLY PROJECT ~ Guava Fruit Fly (Bactrocera correcta) Host List Scientific Name Common Name Acca sellowiana Pineapple-guava, feijoa Anacardium occidentale Cashew,el anacardo Annona cherimola Cherimoya, custard-apple Annona muricata Soursop, araticum-grande Arecu catechu Areca palm, Areca Artocarpus chama Chaplash Artocarpus integer Chempedak Averrhoa carambola Carambola (star fruit), Fruta estrella Baccaurea racemosa Menteng Bellucia dichotoma N/A Bellucia grossularioides N/A Benincasa hispida Ash Gourd, calabaza de la ceniza Bouea macrophylla Gandaria Bouea oppositifolia Mariantree Byrsonima crassifolia Craboo, golden-spoon Calycolpus moritzianus N/A Campomanesia lineatifolia Guabiroba, guayaba de leche Capparis sepiaria Wild Caper Bush Capparis thorelii Cap Thorel, Thorel casquillo Careya arborea Slow Match Tree, Slow Árbol Partido Careya sphaerica Kra Doon Carica papaya Papaya Carissa carandas Bengal Currants, Bengala Pasas Citrus maxima Pomelo Citrus reticulata Mandarin Citrus sinensis Sweet Orange Citrus xsinensis Sweet orange, blood orange Citrus xtangelo Tangelo, uglifruit Updated 7/6/20 - cdfa USDA ------ ~--- CALIFORNIA EXOTIC FRUIT FLY PROJECT ~ Guava Fruit Fly (Bactrocera correcta) Host List Scientific Name Common Name Clausena lansium Wampi Coccinia grandis Ivy Gourd, Ivy calabaza Coffea arabica Arabica coffee, Arabian coffee Coffea canephora Robusta Coffee, café Robusta Couma utilis Sorva, sorva pequena Cucumis melo Melon Dimocarpus longan Longan Diospyros digyna Black persimmon, -

Plantimmigrants
729 PLANT IMMIGRANTS Issued monthly by the Office of Foreign Seed and Plant Introduction, Bureau of Plant Industry, Department of Agriculture. No. 93. January 1914. Genera Represented in This Number, Bambos 37009 Forsythla 37004 Bromelia 36967 Garcirvia 36977 Citrus 36942-951 Glycine 37036-037 36971-975 37040-055 Crotalaria 36969 Holcus 36960-963 Cudrania 36986 Myrciaria 37034 Elaeis 36973 Sponaias 37018 Eugenia 36968, 37017 37026 PLATES: Eugenia luschnathiana. The Pitomba of Brazil. (NOTE: Applications for material listed in this bulletin may be made at any time to this Office. As they are received they are filed, and when the material is ready for the use of experimenters it is sent to those on the list of applicants who can show that they are prepared to care for it, as well as to others selected because of their special < fitness to experiment with the particular plants imported. One of the main objects of the Office of Foreign Seed and Plant Introduction is to secure material for plant experimenters, and it will undertake as far as possible to fill 'any specific requests for foreign seeds or plants from plant breeders or others interested.) 730 Banibos guadita. (Poaceae.) 57003. Seeds of a bamboo from Puerto Bertoni, Paraguay. Presented by Mr. G. H. Bertoni. "Takuara, a native Paraguayan bamboo, which grows by preference in the low sandy lands along the rivers. Here ib reaches a height of 15 to 20 m. (50-65 ft.) and the culm which reaches a diameter of from 10 to 15 cm. (4-6 in.) is used for pots or jars." (Bertoni.) For distribution later. -

Chemical Composition, Antioxidant, Antimicrobial Activity, Toxicity, Genetic Analysis and Popular Use of Eugenia Luschnathiana (O
BOLETIN LATINOAMERICANO Y DEL CARIBE DE PLANTAS MEDICINALES Y AROMÁTICAS © / ISSN 0717 7917 / www.blacpma.ms-editions.cl Revisión / Review Chemical composition, antioxidant, antimicrobial activity, toxicity, genetic analysis and popular use of Eugenia luschnathiana (O. Berg) Klotzsch ex B. D. Jacks: a literature review [Composición química, actividad antioxidante, antimicrobiana, toxicidad, análisis genético y uso popular de Eugenia luschnathiana (O. Berg) Klotzsch ex B. D Jack: una revisión de la literatura] Martha Quitéria Silva Henriques, David Henrique Xavier Barbosa, Danielle da Nóbrega Alves, Ana Karoline Vieira Melo & Ricardo Dias de Castro Health Sciences Center, Federal University of Paraiba, João Pessoa, Paraíba, Brazil Abstract: This review describes the geographical distribution, botanical data, popular use, chemical composition, pharmacological activities and genetic aspects related to Eugenia luschnathiana, a native Reviewed by: Brazilian plant popularly known as “bay pitomba”. E. luschnathiana leaves are characterized Marcelo Luis Wagner morphologically by the presence of a petiole, an attenuated base, acuminated apex, elliptical shape, and Universidad de Buenos Aires parallel venation. The major chemical compounds found in E. luschnathiana are sesquiterpenes. Literature Argentina reports showed that E. luschnathiana extracts have antioxidant properties and antimicrobial activity against Gram-negative and Gram-positive bacteria. The extracts from the leaf, fruit and stem, and a Ali Parlar concentrated residual solution of its essential oil, displayed negligible toxicity. Lastly, a cytogenetic University of Adiyaman Turkey analysis indicated that some markers can be used for the study of genetic diversity, population structure, and genetic improvements. The information available on E. luschnathiana supports the hypothesis that this plant may be a source of compounds with promising pharmacological activity. -

1 CV: Snow 2018
1 NEIL SNOW, PH.D. Curriculum Vitae CURRENT POSITION Associate Professor of Botany Curator, T.M. Sperry Herbarium Department of Biology, Pittsburg State University Pittsburg, KS 66762 620-235-4424 (phone); 620-235-4194 (fax) http://www.pittstate.edu/department/biology/faculty/neil-snow.dot ADJUNCT APPOINTMENTS Missouri Botanical Garden (Associate Researcher; 1999-present) University of Hawaii-Manoa (Affiliate Graduate Faculty; 2010-2011) Au Sable Institute of Environmental Studies (2006) EDUCATION Ph.D., 1997 (Population and Evolutionary Biology); Washington University in St. Louis Dissertation: “Phylogeny and Systematics of Leptochloa P. Beauv. sensu lato (Poaceae: Chloridoideae)”. Advisor: Dr. Peter H. Raven. M.S., 1988 (Botany); University of Wyoming. Thesis: “Floristics of the Headwaters Region of the Yellowstone River, Wyoming”. Advisor: Dr. Ronald L. Hartman B.S., 1985 (Botany); Colorado State University. Advisor: Dr. Dieter H. Wilken PREVIOUS POSITIONS 2011-2013: Director and Botanist, Montana Natural Heritage Program, Helena, Montana 2007-2011: Research Botanist, Bishop Museum, Honolulu, Hawaii 1998-2007: Assistant then Associate Professor of Biology and Botany, School of Biological Sciences, University of Northern Colorado 2005 (sabbatical). Project Manager and Senior Ecologist, H. T. Harvey & Associates, Fresno, CA 1997-1999: Senior Botanist, Queensland Herbarium, Brisbane, Australia 1990-1997: Doctoral student, Washington University in St. Louis; Missouri Botanical Garden HERBARIUM CURATORIAL EXPERIENCE 2013-current: Director -

Vermicomposting of Green Eucalyptus Leaf Litter by Eisenia Foetida and Eudrilus Eugenia Miss
International Journal of Environment, Agriculture and Biotechnology (IJEAB) Vol-2, Issue-6, Nov -Dec- 2017 http://dx.doi.org/10.22161/ijeab/2.6.6 ISSN: 2456-1878 Vermicomposting of green Eucalyptus leaf litter by Eisenia foetida and Eudrilus eugenia Miss. Ritu Nagar1*, Dr. Anurag Titov2, Dr. Praveesh Bhati3 1,2Department of Botany, Govt. Madhav Science PG College,Ujjain,(M.P.)India. 3Department of Microbiology, Govt. Madhav Science PG College,Ujjain,(M.P.),India. *corresponding author Abstract— Effective clearance of different types of waste is a microbiologically active organic material formed has become significant to sustain healthy environment. from the interactions between earthworms and different Vermicomposting has become a suitable substitute for the type of microorganisms (Domínguez, 2004).Through the safe, hygienic and cost effective disposal of organic solid vermicomposting process, environmental risk of leaf wastes. Earthworms decompose organic waste leading to waste material reduces by transforming into a safer and the production of compost which is high in nutrient more stable product suitable for application to soil content. The present work has been designed to reveal (Lazcano et al., 2008), and also reduces the transportation competitive and / or beneficial interactions by studying costs because of the significant reduction in the water the inter-specific interactions in terms of growth, content of the raw organic matter. Composted materials maturation, survival and vermicomposting efficiency of are therefore gaining acceptance as organic fertilizers in two earthworm species Eisenia foetida and Eudrilus sustainable agriculture, and there has been a considerable eugenia exposed to green leaf litter of Eucalyptus and increase in research dedicated to the study of the effects measured physical variables during entire process. -

(GISD) 2021. Species Profile Eugenia Uniflora. Available
FULL ACCOUNT FOR: Eugenia uniflora Eugenia uniflora System: Terrestrial Kingdom Phylum Class Order Family Plantae Magnoliophyta Magnoliopsida Myrtales Myrtaceae Common name Cayennekirsche (German), Surinaamsche kersh (English, Surinam), cerisier de Cayenne (French), Florida cherry (English), cayenne cherry (English), pitanga (Spanish), Brazilian cherry (English), cerises-cotes (English, Guadeloupe), cerese à côtes (English, Guadeloupe), nagapiry (Spanish), cerezo de Cayena (Spanish), pitanga-da-praia (Portuguese), Surinamkirsche (German), guinda (English, El Salvador), cereza quadrada (English, Colombia), pendanga (English, Venezuela), monkie monkie kersie (English, Surinam), cerise de pays (English, French Guiana), cerise de Cayenne (English, French Guiana), zoete kers (English, Surinam), Surinam cherry (English), cerise carée (English, French Guiana), French cherry (English), Barbados cherry (English), red Brazil cherry (English), kafika papalangi (English), kafika palangi (English), kafika (English), menemene (English), venevene (English), ñanga-piré (English, Argentina), cerisier carré (French) Synonym Eugenia michelii , Lam. Eugenia brasiliana , (L.) Aubl. Myrtus brasiliana , L. Myrtus brasiliana , L. var. normalis Kuntze Plinia pedunculata , L.f. Plinia rubra , L. Stenocalyx michelii , O. Berg Stenocalyx uniflorus , (L.) Kausel Similar species Eugenia Summary Eugenia uniflora is an evergreen shrub that can reach tree like proportions. It is a hardy species that can thrive in a variety of habitats, both in its native and introduced