Integrating Birds Into Sagebrush Management Version 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vulnerability Assessment of Sagebrush Ecosystems: Four Corners and Upper Rio Grande Regions of The
Vulnerability Assessment of Sagebrush Ecosystems: Four Corners and Upper Rio Grande Regions of the Southern Rockies Landscape Conservation Cooperative Prepared by Mary I. Williams and Megan M. Friggens United States Forest Service Rocky Mountain Research Station Albuquerque, New Mexico December 29, 2017 1 Cover Photo Credit: Mary Williams, 2010 Mary Williams is an Ecologist with the Nez Perce Tribe, Lapwai, ID Megan Friggens is a Research Ecologist with the USDA Forest Service, Rocky Mountain Research Station This analysis was prepared in partial fulfillment of agreements between Rocky Mountain Research Station (RMRS) and the Southern Rockies Landscape Conservation Cooperative (SRLCC: FWS #15-IA-011221632-164 and BOR #15-IA-11221632-163). This is one in a series of four assessments produced for focal landscapes within the SRLCC. Data associated with this assessment can be found at the SRLCC Conservation Planning Atlas web portal: https://srlcc.databasin.org Acknowledgements: Kristin Peltz, USFS, contributed to early drafts of this report. Stephanie Mueller, Northern Arizona University, processed many of the spatial datasets. We thank the participants of the 2016 and 2017 Adaptation Forum’s held in Durango, CO, and Albuquerque and Taos, NM. For further questions or comments, contact: [email protected] 2 Contents Purpose........................................................................................................................................ 6 Background ................................................................................................................................. -

The Abundance of Greater Sage-Grouse As a Proxy for the Abundance of Sagebrush-Associated Songbirds in Wyoming, USA
VOLUME 15, ISSUE 2, ARTICLE 16 Carlisle, J. D., and A. D. Chalfoun. 2020. The abundance of Greater Sage-Grouse as a proxy for the abundance of sagebrush-associated songbirds in Wyoming, USA. Avian Conservation and Ecology 15(2):16. https://doi.org/10.5751/ACE-01702-150216 Copyright © 2020 by the author(s). Published here under license by the Resilience Alliance. Research Paper The abundance of Greater Sage-Grouse as a proxy for the abundance of sagebrush-associated songbirds in Wyoming, USA Jason D. Carlisle 1,2 and Anna D. Chalfoun 3 1Wyoming Cooperative Fish and Wildlife Research Unit, Department of Zoology and Physiology, Program in Ecology, University of Wyoming, 2Western EcoSystems Technology, Inc., 3U.S. Geological Survey Wyoming Cooperative Fish and Wildlife Research Unit, Department of Zoology and Physiology and Program in Ecology, University of Wyoming ABSTRACT. Surrogate-species concepts are prevalent in animal conservation. Such strategies advocate for conservation by proxy, wherein one species is used to represent other taxa to obtain a conservation objective. The efficacy of such approaches has been rarely assessed empirically, but is predicated on concordance between the surrogate and sympatric taxa in distribution, abundance, and ecological requirements. Our objective was to identify whether the abundance of a high-profile umbrella species (Greater Sage-Grouse, Centrocercus urophasianus, hereafter sage-grouse) was associated with the abundance of six other members of the avian community for which it is presumed to be a surrogate, including three sagebrush-obligate and three sagebrush-associated songbird species. We predicted that sage-grouse abundance would align most closely with the breeding abundance of other sagebrush-obligate birds. -

Sagebrush Sparrow Artemisiospiza Nevadensis
Wyoming Species Account Sagebrush Sparrow Artemisiospiza nevadensis REGULATORY STATUS USFWS: Migratory Bird USFS R2: Sensitive USFS R4: No special status Wyoming BLM: Sensitive State of Wyoming: Protected Bird CONSERVATION RANKS USFWS: Bird of Conservation Concern WGFD: NSS4 (Bc), Tier II WYNDD: G5, S3S4 Wyoming Contribution: HIGH IUCN: Least Concern PIF Continental Concern Score: 11 STATUS AND RANK COMMENTS The Wyoming Natural Diversity Database has assigned Sagebrush Sparrow (Artemisiospiza nevadensis) a state conservation rank ranging from S3 (Vulnerable) to S4 (Apparently Secure) because of uncertainty about the abundance and population trends of this species in Wyoming. NATURAL HISTORY Taxonomy: In 2013, Sage Sparrow (Artemisiospiza belli, previously Amphispiza belli 1) was split into two species based on genetic evidence and differences in ecology and morphology: Sagebrush Sparrow (Artemisiospiza nevadensis) and Bell’s Sparrow (Artemisiospiza belli) 2, 3. Only Sagebrush Sparrow is found in Wyoming. Due to the extremely recent nature of this taxonomic revision, most of the references cited in this account refer to Sage Sparrow as it was recognized before the split. There are currently no recognized subspecies of Sagebrush Sparrow 4. Description: Identification of Sagebrush Sparrow is possible in the field. Adults weigh between 15.3–21.9 g, range in length from 12.1–15.0 cm, and have a wingspan of approximately 21.0 cm 3, 5. The sexes are similar in appearance, but males are larger than females 3. Adults have a pale grey head; black eyes with a complete white eye ring; white spots above the lores; white malars; gray bill; white throat and whitish underparts with an isolated dark spot on the breast; pale grayish brown upperparts with dark streaking on the mantle; dark brown tail; and brown legs 3, 5. -

AOU Check-List Supplement
AOU Check-list Supplement The Auk 117(3):847–858, 2000 FORTY-SECOND SUPPLEMENT TO THE AMERICAN ORNITHOLOGISTS’ UNION CHECK-LIST OF NORTH AMERICAN BIRDS This first Supplement since publication of the 7th Icterus prosthemelas, Lonchura cantans, and L. atricap- edition (1998) of the AOU Check-list of North American illa); (3) four species are changed (Caracara cheriway, Birds summarizes changes made by the Committee Glaucidium costaricanum, Myrmotherula pacifica, Pica on Classification and Nomenclature between its re- hudsonia) and one added (Caracara lutosa) by splits constitution in late 1998 and 31 January 2000. Be- from now-extralimital forms; (4) four scientific cause the makeup of the Committee has changed sig- names of species are changed because of generic re- nificantly since publication of the 7th edition, it allocation (Ibycter americanus, Stercorarius skua, S. seems appropriate to outline the way in which the maccormicki, Molothrus oryzivorus); (5) one specific current Committee operates. The philosophy of the name is changed for nomenclatural reasons (Baeolo- Committee is to retain the present taxonomic or dis- phus ridgwayi); (6) the spelling of five species names tributional status unless substantial and convincing is changed to make them gramatically correct rela- evidence is published that a change should be made. tive to the generic name (Jacamerops aureus, Poecile The Committee maintains an extensive agenda of atricapilla, P. hudsonica, P. cincta, Buarremon brunnein- potential action items, including possible taxonomic ucha); (7) one English name is changed to conform to changes and changes to the list of species included worldwide use (Long-tailed Duck), one is changed in the main text or the Appendix. -

An Abstract of the Thesis Of
AN ABSTRACT OF THE THESIS OF Vanessa Schroeder for the degree of Master of Science in Wildlife Science presented on December 3, 2020. Title: The Role of Grazing and Weather in Nest Predator-Prey Dynamics and Reproductive Success of Sagebrush-obligate Songbirds Abstract approved: ________________________________________________________________ Jonathan B. Dinkins, W. Douglas Robinson Livestock grazing occurs worldwide, spanning over 25% of land globally. Effective conservation of biodiversity relies upon understanding the interactions of agricultural management practices and increasingly variable weather associated with climate change. I evaluated grazing, weather and predator-prey interactions within a grazing experiment in the sagebrush ecosystem of southeastern Oregon. I studied key weather variables, nest predator activity and the nest success rates of two species of sagebrush-obligate songbirds, Brewer’s sparrow (Spizella breweri) and sagebrush sparrow (Artemisiospiza nevadensis) under dormant season grazing, rotational grazing and a no-graze control. I found that while weather was an important factor explaining nest success for both songbird species, nest success for sagebrush sparrows increased in grazed pastures relative to no grazing. Grazing influenced the nest predator community of these songbirds and was likely one mechanism explaining the increase in sagebrush sparrow nest success relative to a no-graze control. Nest predator activity was lower for most predators in rotationally grazed pastures relative to no grazing, and lower rodent and badger activity was associated with higher songbird nest success. Ten species, predominately birds and snakes were documented on camera depredating failed Brewer’s sparrow and sagebrush sparrow nests. My results suggest that reductions in screening cover caused by grazing within the range of reductions observed in my study do not present a threat to these birds, and conservation should focus on management to mitigate the negative effects of extreme weather on the sagebrush ecosystem and associated wildlife. -
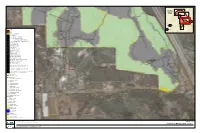
Map FIGURE 11A
Index Map FIGURE 11A FIGURE 11B FIGURE 11C FIGURE 11D FIGURE 11D FIGURE 11D Project Site FIGURE 11D On-site Off-site Vegetation Communities AGR, Agriculture CLOW, Coast live oak woodland CSS, Diegan coastal sage scrub CSS-CHP, Coastal sage - chaparral transition CSSB, Coastal sage scrub - Baccharis dominated DEV, Urban/developed DH, Disturbed habitat DW, Disturbed Wetland EAGR, Agriculture EUC, Eucalyptus woodland FWM, Freshwater marsh IAGR, Intensive agriculture MFS, Mulefat scrub NNG, Non-native grassland NNW, Non-native Woodland ORC, Orchard and vineyards ORF, Southern coast live oak riparian forest SMX, Southern mixed chaparral SOC, Scrub oak chaparral SWS, Southern willow scrub SWS/TS, Southern willow scrub/tamarisk scrub dBSC, Flat-topped buckwheat - disturbed dCLOW, Coast Live Oak Woodland - disturbed dCSS, Diegan coastal sage scrub - disturbed dCSSB, Coastal sage scrub - Baccharis dominated - disturbed dSMX, Southern mixed chaparral - disturbed Oak Root Zones Las Posas Soil Series Wildlife Species Coastal California gnatcatcher Nuttall’s woodpecker Oak titmouse Red-shouldered hawk Sharp-shinned hawk Yellow warbler Desert woodrat (midden) Blainville’s horned lizard Blainville’s horned lizard (scat) Coast patch-nosed snake Coastal whiptail Red diamond rattlesnake Plant Species Engelmann oak Munz’s sage Ramona horkelia Summer holly ashy spike-moss chaparral rein orchid Engelmann oak Orcutt’s brodiaea Ramona horkelia Summer holly Project Impacts Permanent Impact Temporary Impact Temporary Construction Easement Open Space SOURCE: SANDAG -

Mesa Wind Repower Project, Appendix G, Biological Resources
Appendix G Biological Resources Technical Report APPENDIX G Biological Resources Technical Report Mesa Wind Project Repower Prepared for: PMB 422 6703 Oak Creek Rd. Mojave, CA 93501 Prepared by: 5020 Chesebro Road, Suite 200 Agoura Hills, CA 91301 February 2020 Biological Resources Technical Report Mesa Wind Project Repower Contents 1.0 Introduction ................................................................................................................................................................................................................. 1 1.1 Project Description .................................................................................................................................................................................... 1 1.2 Project Location ........................................................................................................................................................................................... 2 2.0 Methods ........................................................................................................................................................................................................................... 3 2.1 Literature Review ........................................................................................................................................................................................ 3 2.2 Field Surveys .................................................................................................................................................................................................. -

2.4 Biological Resources 2.4 Biological Resources the Descriptions and Evaluation of Biological Resources Impacts in This Sectio
2.4 Biological Resources 2.4 Biological Resources The descriptions and evaluation of biological resources impacts in this section are based on information compiled from the Biological Resources Technical Report (BTR) prepared for the proposed Newland Sierra Project (project) by Dudek (Appendix H). This section of the EIR will (1) describe the existing conditions of biological resources within the project Site in terms of vegetation, jurisdictional resources, flora, wildlife, and wildlife habitats; (2) discuss potential impacts to biological resources that would result from development of the Site and describe those impacts in terms of biological significance in view of federal, state, and local laws and policies; and (3) recommend mitigation measures for potential impacts to sensitive biological resources, if necessary. Recommendations will follow federal, state, and local rules and regulations, including the California Environmental Quality Act (CEQA), the County of San Diego’s (County) Guidelines for Determining Significance and Report Format and Contents Requirements: Biological Resources (County of San Diego 2010a, 2010b), and the County’s Resource Protection Ordinance (RPO) (County of San Diego 2007). Comments received in response to the Notice of Preparation (NOP) included concerns regarding listed and other sensitive species, wildlife corridor/movement areas, conflicts between human and wildlife interface, fuel modification zones, nesting birds, and the creation of a sub-regional preserve system. These concerns are addressed and summarized in this section. A copy of the NOP and comment letters received in response to the NOP are included in Appendix A of this EIR. 2.4.1 Proposed Open Space Design On-Site Open Space The biological open space for the proposed project would include three large, interconnected, open space blocks within the project Site, as well as a large off-site biological open space parcel. -

Status of 10 Bird Species of Conservation Concern, Vol. 2
STATUS OF 10 ADDITIONAL BIRD SPECIES OF CONSERVATION CONCERN IN US FISH & WILDLIFE SERVICE REGION 6 Final Report to: United States Fish & Wildlife Service, Region 6 Denver, Colorado By Christopher J. Butler, Ph.D., Jeffrey B. Tibbits and Katrina Hucks Department of Biology University of Central Oklahoma Edmond, Oklahoma 73034-5209 January 20, 2014 1 Table of Contents American Bittern (Botaurus lentiginosus) ..................................................................................................... 3 Ferruginous Hawk (Buteo regalis) ............................................................................................................... 12 Black Rail (Laterallus jamaicensis) .............................................................................................................. 20 Wilson’s Phalarope (Phalaropus tricolor) ................................................................................................... 27 Lewis’s Woodpecker (Melanerpes lewis) .................................................................................................... 34 Olive-sided Flycatcher (Contopus cooperi) ................................................................................................. 41 Sedge Wren (Cistothorus platensis) ............................................................................................................ 48 Chestnut-collared Longspur (Calcarius ornatus)......................................................................................... 56 Brewer’s Sparrow (Spizella breweri) -

SAFE North American Songbirds Action Plan (August 2020- August 2023)
SAFE North American Songbirds Action Plan (August 2020- August 2023) 1 | P a g e Table of Contents Program Goal 3 Program Operating Structure 4 Conservation Target 8 Status of songbirds Within AZA 10 Origin of SAFE North American Songbird 10 AZA Conservation & Public Engagement 11 Conservation Status of North American Songbirds 13 Recovery Plan or Other Conservation Plan In Effect 15 Threats To Native Songbirds 16 SAFE NAS Program Objectives and Actions 25 Conservation Objectives 20 Public/Stakeholder Engagement Objectives 21 Communication/Public Awareness Objectives 22 Fundraising Objectives 23 Actions 30 References 34 Appendix I: List & Status of North American Songbirds 36 Appendix II: SAFE NAS Program Partner Activities 50 Appendix III: SAFE NAS Endorsement Policy 59 APPENDIX IV: SAFE NAS Social Media Policy 61 2 | P a g e The 2020-2023 SAFE North American Songbirds (NAS) Action Plan was developed by the SAFE NAS Steering committee consisting of leaders from 12 AZA member institutions (Akron Zoological Park, Brevard Zoo, Columbus Zoo and Aquarium, Detroit Zoological Society, Fort Wayne Children’s Zoo, North Carolina Aquarium on Roanoke Island, Riverbanks Zoo & Garden, Saint Louis Zoo, Smithsonian’s National Zoo & Conservation Biology Institute, The Wilds, Toronto Zoo and Zoo Miami,) along with project partners and external advisors. The SAFE North American Songbird program invites and welcomes all interested zoos and aquariums to become program partners, funders and collaborators in our efforts to save and protect North American songbirds. Program Goal The goal of Saving Animals From Extinction North American Songbirds (SAFE NAS) is to reduce the threats to North American songbirds and secure sustainable wild populations of these species throughout their ranges. -

Status Assessment and Conservation Plan for the Grasshopper Sparrow (Ammodramus Savannarum)
U.S. Fish & Wildlife Service Status Assessment and Conservation Plan for the Grasshopper Sparrow (Ammodramus savannarum) ©Robert Royse 2006 Status Assessment and Conservation Plan for the Grasshopper Sparrow (Ammodramus savannarum) Version 1.0 2015 prepared by Janet M. Ruth U.S. Geological Survey Fort Collins Science Center USGS Arid Lands Field Station UNM Biology Department MSC03 2020, 1 University of New Mexico Albuquerque, NM 87131-0001 [email protected] Recommended Citation Ruth, J.M. 2015. Status Assessment and Conservation Plan for the Grasshopper Sparrow (Ammodramus savannarum). Version 1.0 U.S. Fish and Wildlife Service, Lakewood, Colorado. 109 pp. Cover photo provided by Robert Royse Acknowledgements This project was funded by the U.S. Fish and Wildlife Service (USFWS), Migratory Bird Program, Region 6. Thanks to T. Lickfett (USFWS) for his assistance in creating the range map (Figure 1) from BirdLife International and NatureServe data; to B.W. Smith (USFWS), R. Doster (USFWS), R. Russell (USFWS), A. Forbes (USFWS), P. Vickery (Center for Ecological Research), and J. Herkert (Illinois DNR) for reviews of previous versions of this document. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Table of Contents Executive Summary ....................................................................................................................................... 1 I. Introduction .............................................................................................................................................. -

Download the Booklet
Mammals BEARS EARS NATIONAL MONUMENT WILDLIFE Highlighted in the 2016 PROCLAMATION PREPARED BY JONATHAN BARTH & THOMAS MEINZEN Tim Peterson “ The diverse vegetation and topography of the Bears Ears area, in turn, support a variety of wildlife species . Protection of the Bears Ears area will preserve its . diverse array of natural and scientific resources, ensuring that the . scientific values of this area remain for the benefit of all Americans. NOW, THEREFORE, I, BARACK OBAMA . hereby proclaim the objects identified above that are situated upon lands and interests in lands owned or controlled by the Federal Government to be the Bears Ears National Monument.” Excerpts from Bears Ears National Monument Proclamation, December 28, 2016 1 cover photo: Bears Ears from Valley of the Gods, by Tim Peterson cover photo inset left to right: James Marvin Phelps, Thomas Meinzen, Greg Schechter, National Park Service, Steve Jurvetson Seventy-six WILDLIFE SPECIES were noted in the Proclamation that established Bears Ears National Monument. This booklet, as well as the Proclamation, are intended to help all of us acknowledge wildlife as one of the many values Bears Ears National Monument offers our nation. The wildlife species are grouped first by class (mammal, amphibian, etc.), then by large and small animals (within acknowledgements mammal, bird and reptile classes), then by family (cat family, raptor family, etc.) and subsequently by common name. Jonathan Barth and Thomas Meinzen, skilled volunteer Mammals (3o species) and intern (respectively) have compiled this booklet with Page 5 the guidance of Mary O’Brien. Many photographers (listed below each photo) have generously given permission for Birds (25 species) Page 10 their images to be used; a number of which are available under the “creative commons”: https://creativecommons.