A Harmine-Derived Beta-Carboline Displays Anti-Cancer Effects in Vitro by Targeting Protein Synthesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplementary Materials: Evaluation of Cytotoxicity and Α-Glucosidase Inhibitory Activity of Amide and Polyamino-Derivatives of Lupane Triterpenoids
Supplementary Materials: Evaluation of cytotoxicity and α-glucosidase inhibitory activity of amide and polyamino-derivatives of lupane triterpenoids Oxana B. Kazakova1*, Gul'nara V. Giniyatullina1, Akhat G. Mustafin1, Denis A. Babkov2, Elena V. Sokolova2, Alexander A. Spasov2* 1Ufa Institute of Chemistry of the Ufa Federal Research Centre of the Russian Academy of Sciences, 71, pr. Oktyabrya, 450054 Ufa, Russian Federation 2Scientific Center for Innovative Drugs, Volgograd State Medical University, Novorossiyskaya st. 39, Volgograd 400087, Russian Federation Correspondence Prof. Dr. Oxana B. Kazakova Ufa Institute of Chemistry of the Ufa Federal Research Centre of the Russian Academy of Sciences 71 Prospeсt Oktyabrya Ufa, 450054 Russian Federation E-mail: [email protected] Prof. Dr. Alexander A. Spasov Scientific Center for Innovative Drugs of the Volgograd State Medical University 39 Novorossiyskaya st. Volgograd, 400087 Russian Federation E-mail: [email protected] Figure S1. 1H and 13C of compound 2. H NH N H O H O H 2 2 Figure S2. 1H and 13C of compound 4. NH2 O H O H CH3 O O H H3C O H 4 3 Figure S3. Anticancer screening data of compound 2 at single dose assay 4 Figure S4. Anticancer screening data of compound 7 at single dose assay 5 Figure S5. Anticancer screening data of compound 8 at single dose assay 6 Figure S6. Anticancer screening data of compound 9 at single dose assay 7 Figure S7. Anticancer screening data of compound 12 at single dose assay 8 Figure S8. Anticancer screening data of compound 13 at single dose assay 9 Figure S9. Anticancer screening data of compound 14 at single dose assay 10 Figure S10. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Gene Expression Profiling Analysis Contributes to Understanding the Association Between Non-Syndromic Cleft Lip and Palate, and Cancer
2110 MOLECULAR MEDICINE REPORTS 13: 2110-2116, 2016 Gene expression profiling analysis contributes to understanding the association between non-syndromic cleft lip and palate, and cancer HONGYI WANG, TAO QIU, JIE SHI, JIULONG LIANG, YANG WANG, LIANGLIANG QUAN, YU ZHANG, QIAN ZHANG and KAI TAO Department of Plastic Surgery, General Hospital of Shenyang Military Area Command, PLA, Shenyang, Liaoning 110016, P.R. China Received March 10, 2015; Accepted December 18, 2015 DOI: 10.3892/mmr.2016.4802 Abstract. The present study aimed to investigate the for NSCL/P were implicated predominantly in the TGF-β molecular mechanisms underlying non-syndromic cleft lip, signaling pathway, the cell cycle and in viral carcinogenesis. with or without cleft palate (NSCL/P), and the association The TP53, CDK1, SMAD3, PIK3R1 and CASP3 genes were between this disease and cancer. The GSE42589 data set found to be associated, not only with NSCL/P, but also with was downloaded from the Gene Expression Omnibus data- cancer. These results may contribute to a better understanding base, and contained seven dental pulp stem cell samples of the molecular mechanisms of NSCL/P. from children with NSCL/P in the exfoliation period, and six controls. Differentially expressed genes (DEGs) were Introduction screened using the RankProd method, and their potential functions were revealed by pathway enrichment analysis and Non-syndromic cleft lip, with or without cleft palate (NSCL/P) construction of a pathway interaction network. Subsequently, is one of the most common types of congenital defect and cancer genes were obtained from six cancer databases, and affects 3.4-22.9/10,000 individuals worldwide (1). -

Transcriptomic and Epigenomic Characterization of the Developing Bat Wing
ARTICLES OPEN Transcriptomic and epigenomic characterization of the developing bat wing Walter L Eckalbar1,2,9, Stephen A Schlebusch3,9, Mandy K Mason3, Zoe Gill3, Ash V Parker3, Betty M Booker1,2, Sierra Nishizaki1,2, Christiane Muswamba-Nday3, Elizabeth Terhune4,5, Kimberly A Nevonen4, Nadja Makki1,2, Tara Friedrich2,6, Julia E VanderMeer1,2, Katherine S Pollard2,6,7, Lucia Carbone4,8, Jeff D Wall2,7, Nicola Illing3 & Nadav Ahituv1,2 Bats are the only mammals capable of powered flight, but little is known about the genetic determinants that shape their wings. Here we generated a genome for Miniopterus natalensis and performed RNA-seq and ChIP-seq (H3K27ac and H3K27me3) analyses on its developing forelimb and hindlimb autopods at sequential embryonic stages to decipher the molecular events that underlie bat wing development. Over 7,000 genes and several long noncoding RNAs, including Tbx5-as1 and Hottip, were differentially expressed between forelimb and hindlimb, and across different stages. ChIP-seq analysis identified thousands of regions that are differentially modified in forelimb and hindlimb. Comparative genomics found 2,796 bat-accelerated regions within H3K27ac peaks, several of which cluster near limb-associated genes. Pathway analyses highlighted multiple ribosomal proteins and known limb patterning signaling pathways as differentially regulated and implicated increased forelimb mesenchymal condensation in differential growth. In combination, our work outlines multiple genetic components that likely contribute to bat wing formation, providing insights into this morphological innovation. The order Chiroptera, commonly known as bats, is the only group of To characterize the genetic differences that underlie divergence in mammals to have evolved the capability of flight. -

EIF1AX and RAS Mutations Cooperate to Drive Thyroid Tumorigenesis Through ATF4 and C-MYC
Published OnlineFirst October 10, 2018; DOI: 10.1158/2159-8290.CD-18-0606 RESEARCH ARTICLE EIF1AX and RAS Mutations Cooperate to Drive Thyroid Tumorigenesis through ATF4 and c-MYC Gnana P. Krishnamoorthy 1 , Natalie R. Davidson 2 , Steven D. Leach 1 , Zhen Zhao 3 , Scott W. Lowe 3 , Gina Lee 4 , Iňigo Landa 1 , James Nagarajah 1 , Mahesh Saqcena 1 , Kamini Singh 3 , Hans-Guido Wendel3 , Snjezana Dogan 5 , Prasanna P. Tamarapu 1 , John Blenis 4 , Ronald A. Ghossein 5 , Jeffrey A. Knauf 1 , 6 , Gunnar Rätsch 2 , and James A. Fagin 1 , 6 ABSTRACT Translation initiation is orchestrated by the cap binding and 43S preinitiation com- plexes (PIC). Eukaryotic initiation factor 1A (EIF1A) is essential for recruitment of the ternary complex and for assembling the 43S PIC. Recurrent EIF1AX mutations in papillary thyroid cancers are mutually exclusive with other drivers, including RAS . EIF1AX mutations are enriched in advanced thyroid cancers, where they display a striking co-occurrence with RAS , which cooperates to induce tumorigenesis in mice and isogenic cell lines. The C-terminal EIF1AX-A113splice mutation is the most prevalent in advanced thyroid cancer. EIF1AX-A113splice variants stabilize the PIC and induce ATF4, a sensor of cellular stress, which is co-opted to suppress EIF2α phosphorylation, enabling a gen- eral increase in protein synthesis. RAS stabilizes c-MYC, an effect augmented by EIF1AX-A113splice. ATF4 and c-MYC induce expression of amino acid transporters and enhance sensitivity of mTOR to amino acid supply. These mutually reinforcing events generate therapeutic vulnerabilities to MEK, BRD4, and mTOR kinase inhibitors. SIGNIFICANCE: Mutations of EIF1AX, a component of the translation PIC, co-occur with RAS in advanced thyroid cancers and promote tumorigenesis. -

Beyond Traditional Morphological Characterization of Lung
Cancers 2020 S1 of S15 Beyond Traditional Morphological Characterization of Lung Neuroendocrine Neoplasms: In Silico Study of Next-Generation Sequencing Mutations Analysis across the Four World Health Organization Defined Groups Giovanni Centonze, Davide Biganzoli, Natalie Prinzi, Sara Pusceddu, Alessandro Mangogna, Elena Tamborini, Federica Perrone, Adele Busico, Vincenzo Lagano, Laura Cattaneo, Gabriella Sozzi, Luca Roz, Elia Biganzoli and Massimo Milione Table S1. Genes Frequently mutated in Typical Carcinoids (TCs). Mutation Original Entrez Gene Gene Rate % eukaryotic translation initiation factor 1A X-linked [Source: HGNC 4.84 EIF1AX 1964 EIF1AX Symbol; Acc: HGNC: 3250] AT-rich interaction domain 1A [Source: HGNC Symbol;Acc: HGNC: 4.71 ARID1A 8289 ARID1A 11110] LDL receptor related protein 1B [Source: HGNC Symbol; Acc: 4.35 LRP1B 53353 LRP1B HGNC: 6693] 3.53 NF1 4763 NF1 neurofibromin 1 [Source: HGNC Symbol;Acc: HGNC: 7765] DS cell adhesion molecule like 1 [Source: HGNC Symbol; Acc: 2.90 DSCAML1 57453 DSCAML1 HGNC: 14656] 2.90 DST 667 DST dystonin [Source: HGNC Symbol;Acc: HGNC: 1090] FA complementation group D2 [Source: HGNC Symbol; Acc: 2.90 FANCD2 2177 FANCD2 HGNC: 3585] piccolo presynaptic cytomatrix protein [Source: HGNC Symbol; Acc: 2.90 PCLO 27445 PCLO HGNC: 13406] erb-b2 receptor tyrosine kinase 2 [Source: HGNC Symbol; Acc: 2.44 ERBB2 2064 ERBB2 HGNC: 3430] BRCA1 associated protein 1 [Source: HGNC Symbol; Acc: HGNC: 2.35 BAP1 8314 BAP1 950] capicua transcriptional repressor [Source: HGNC Symbol; Acc: 2.35 CIC 23152 CIC HGNC: -
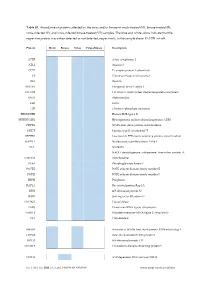
Virus-Infected (V), and Virus-Infected Binase-Treated (VB) Samples
Table S1. Abundance of proteins affected by the virus and/or binase in mock-treated (M), binase-treated (B), virus-infected (V), and virus-infected binase-treated (VB) samples. The blue and white colors indicate that the respective protein was either detected or not detected, respectively, in the sample above 1% FDR cut-off. Protein Mock Binase Virus Virus+Binase Description ACTB Actin, cytoplasmic 1 ATL2 Atlastin-2 CCT7 T-complex protein 1 subunit eta CS Citrate synthase, mitochondrial DES Desmin EEF1A1 Elongation factor 1-alpha 1 EFTUD2 116 kDa U5 small nuclear ribonucleoprotein component ENO1 Alpha-enolase EZR Ezrin GPI Glucose-6-phosphate isomerase HIST1H2BB Histone H2B type 1-B HNRNPA2B1 Heterogeneous nuclear ribonucleoproteins A2/B1 HSPD1 60 kDa heat shock protein, mitochondrial KRT75 Keratin, type II cytoskeletal 75 LRPPRC Leucine-rich PPR motif-containing protein, mitochondrial NAP1L1 Nucleosome assembly protein 1-like 1 NCL Nucleolin NADH dehydrogenase [ubiquinone] iron-sulfur protein 8, NDUFS8 mitochondrial PGK1 Phosphoglycerate kinase 1 POTEE POTE ankyrin domain family member E POTEI POTE ankyrin domain family member I PRPH Peripherin RAP1A Ras-related protein Rap-1A RPS2 40S ribosomal protein S2 SF3B1 Splicing factor 3B subunit 1 TALDO1 Transaldolase TARS Threonine--tRNA ligase, cytoplasmic TARSL2 Probable threonine--tRNA ligase 2, cytoplasmic TKT Transketolase AHSA1 Activator of 90 kDa heat shock protein ATPase homolog 1 HSPA2 Heat shock-related 70 kDa protein 2 RPL15 60S ribosomal protein L15 TXNDC5 Thioredoxin domain-containing protein 5 DDX18 ATP-dependent RNA helicase DDX18 Int. J. Mol. Sci. 2020, 21, x; doi: FOR PEER REVIEW www.mdpi.com/journal/ijms Int. -

Global Gene Expression in Muscle from Fasted/Refed Trout Reveals Up
Rescan et al. BMC Genomics (2017) 18:447 DOI 10.1186/s12864-017-3837-9 RESEARCHARTICLE Open Access Global gene expression in muscle from fasted/refed trout reveals up-regulation of genes promoting myofibre hypertrophy but not myofibre production Pierre-Yves Rescan*, Aurelie Le Cam, Cécile Rallière and Jérôme Montfort Abstract Background: Compensatory growth is a phase of rapid growth, greater than the growth rate of control animals, that occurs after a period of growth-stunting conditions. Fish show a capacity for compensatory growth after alleviation of dietary restriction, but the underlying cellular mechanisms are unknown. To learn more about the contribution of genes regulating hypertrophy (an increase in muscle fibre size) and hyperplasia (the generation of new muscle fibres) in the compensatory muscle growth response in fish, we used high-density microarray analysis to investigate the global gene expression in muscle of trout during a fasting-refeeding schedule and in muscle of control-fed trout displaying normal growth. Results: The compensatory muscle growth signature, as defined by genes up-regulated in muscles of refed trout compared with control-fed trout, showed enrichment in functional categories related to protein biosynthesis and maturation, such as RNA processing, ribonucleoprotein complex biogenesis, ribosome biogenesis, translation and protein folding. This signature was also enriched in chromatin-remodelling factors of the protein arginine N-methyl transferase family. Unexpectedly, functional categories related to cell division and DNA replication were not inferred from the molecular signature of compensatory muscle growth, and this signature contained virtually none of the genes previously reported to be up-regulated in hyperplastic growth zones of the late trout embryo myotome and to potentially be involved in production of new myofibres, notably genes encoding myogenic regulatory factors, transmembrane receptors essential for myoblast fusion or myofibrillar proteins predominant in nascent myofibres. -

Eukaryotic Translation Initiation Factors As Promising Targets in Cancer Therapy
Hao et al. Cell Communication and Signaling (2020) 18:175 https://doi.org/10.1186/s12964-020-00607-9 REVIEW Open Access Eukaryotic translation initiation factors as promising targets in cancer therapy Peiqi Hao1,2†, Jiaojiao Yu1†, Richard Ward3, Yin Liu2, Qiao Hao2,SuAn2* and Tianrui Xu2* Abstract The regulation of the translation of messenger RNA (mRNA) in eukaryotic cells is critical for gene expression, and occurs principally at the initiation phase which is mainly regulated by eukaryotic initiation factors (eIFs). eIFs are fundamental for the translation of mRNA and as such act as the primary targets of several signaling pathways to regulate gene expression. Mis-regulated mRNA expression is a common feature of tumorigenesis and the abnormal activity of eIF complexes triggered by upstream signaling pathways is detected in many tumors, leading to the selective translation of mRNA encoding proteins involved in tumorigenesis, metastasis, or resistance to anti-cancer drugs, and making eIFs a promising therapeutic target for various types of cancers. Here, we briefly outline our current understanding of the biology of eIFs, mainly focusing on the effects of several signaling pathways upon their functions and discuss their contributions to the initiation and progression of tumor growth. An overview of the progress in developing agents targeting the components of translation machinery for cancer treatment is also provided. Keywords: eIF, mRNA translation, Cancer, MAPK, PI3K/Akt, mTOR Background eukaryotes utilize many more initiation factors than do pro- The regulation of gene expression in eukaryotes can occur karyotes, reflecting the greater biological complexity of at different stages including gene transcription and mRNA eukaryotic translation. -

Vaccine-Increased Seq ID Unigene ID Uniprot ID Gene Names
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Vaccine-Increased Seq ID Unigene ID Uniprot ID Gene Names 1_HSPA1A_3303 Hs.274402 P0DMV8 HSPA1A HSP72 HSPA1 HSX70 100_AKAP17A_8227 Hs.522572 Q02040 AKAP17A CXYorf3 DXYS155E SFRS17A XE7 1000_H2AFY_9555 Hs.420272 O75367 H2AFY MACROH2A1 1001_ITPK1_3705 Hs.308122 Q13572 ITPK1 1002_PTPN11_5781 Hs.506852 Q06124 PTPN11 PTP2C SHPTP2 1003_EIF3J_8669 Hs.404056 O75822 EIF3J EIF3S1 PRO0391 1004_TRIP12_9320 Hs.591633 Q14669 TRIP12 KIAA0045 ULF 1006_YEATS2_55689 Hs.632575 Q9ULM3 YEATS2 KIAA1197 1007_SEL1L3_23231 Hs.479384 Q68CR1 SEL1L3 KIAA0746 1008_IDH1_3417 Hs.593422 O75874 IDH1 PICD 101_HSPH1_10808 Hs.36927 Q92598 HSPH1 HSP105 HSP110 KIAA0201 1010_LDLR_3949 Hs.213289 P01130 LDLR 1011_FAM129B_64855 Hs.522401 Q96TA1 NIBAN2 C9orf88 FAM129B 1012_MAP3K5_4217 Hs.186486 Q99683 MAP3K5 ASK1 MAPKKK5 MEKK5 1013_NEFH_4744 Hs.198760 P12036 NEFH KIAA0845 NFH 1014_RAP1B_5908 Hs.369920 P61224 RAP1B OK/SW-cl.11 1015_MCCC1_56922 Hs.47649 Q96RQ3 MCCC1 MCCA 1017_MT1E_4493 Hs.534330 P04732 MT1E 1022_TXNDC5_81567 Hs.150837 Q8NBS9 TXNDC5 TLP46 UNQ364/PRO700 1023_STRA13_201254 Hs.37616 O14503 BHLHE40 BHLHB2 DEC1 SHARP2 STRA13 1024_NPEPPS_9520 Hs.443837 P55786 NPEPPS PSA 1025_YIPF6_286451 Hs.82719 Q96EC8 YIPF6 1026_CLIP1_6249 Hs.524809 P30622 CLIP1 CYLN1 RSN 1027_SRSF7_6432 Hs.309090 Q16629 SRSF7 SFRS7 103_RPS25_6230 Hs.512676 P62851 RPS25 1031_SOCS7_30837 -

Transcriptome Profiles of Porcine Oocytes and Their Corresponding Cumulus Cells Reveal Functional Gene Regulatory Networks
Transcriptome profiles of porcine oocytes and their corresponding cumulus cells reveal functional gene regulatory networks by Katelyn Marie Kimble A thesis submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Master of Science Auburn, Alabama August 4, 2018 Keywords: oocyte, cumulus cells, signaling, transcriptome, porcine Approved by Fernando Biase, Chair, Assistant Professor, Dept. of Animal Sciences Paul Dyce, Assistant Professor, Dept. of Animal Sciences Julie Gard, Professor, Dept. of Clinical Sciences Jacek Wower, Professor, Dept. of Animal Sciences Abstract The oocyte acquires developmental competence as it progresses through folliculogenesis. It does so, by communicating with the surrounding cumulus cells in a bidirectional fashion. Analyses of single oocytes are essential for further clarification of these molecular mechanisms. Standard protocols to obtain RNA for single-cell RNA-Seq involve RNA extraction kits that select for mRNAs or cell lysis procedures, which present limitations when the oocyte is the cell of interest. We adapted the phenol-chloroform procedure for the purification of total RNA from single oocytes with modifications that included the use of Phasemaker™ tubes, a second chloroform wash, and the precipitation of the RNA with glycogen in microcentrifuge tubes. We profiled the total RNA of single oocytes (bovine and porcine), and observed distinct peaks for small RNAs, 18S, and 28S. We amplified the total mRNA and observed DNA fragments longer than 5000 bp in length, suitable for single-cell RNA sequencing. We applied this approach to sequence the transcriptome of 17 prepubertal porcine oocytes and their corresponding cumulus cells. We hypothesized that the transcript profiles of the cumulus cells and oocyte display distinct gene regulatory networks within the oocyte and cumulus cells. -

Mrna Expression in Human Leiomyoma and Eker Rats As Measured by Microarray Analysis
Table 3S: mRNA Expression in Human Leiomyoma and Eker Rats as Measured by Microarray Analysis Human_avg Rat_avg_ PENG_ Entrez. Human_ log2_ log2_ RAPAMYCIN Gene.Symbol Gene.ID Gene Description avg_tstat Human_FDR foldChange Rat_avg_tstat Rat_FDR foldChange _DN A1BG 1 alpha-1-B glycoprotein 4.982 9.52E-05 0.68 -0.8346 0.4639 -0.38 A1CF 29974 APOBEC1 complementation factor -0.08024 0.9541 -0.02 0.9141 0.421 0.10 A2BP1 54715 ataxin 2-binding protein 1 2.811 0.01093 0.65 0.07114 0.954 -0.01 A2LD1 87769 AIG2-like domain 1 -0.3033 0.8056 -0.09 -3.365 0.005704 -0.42 A2M 2 alpha-2-macroglobulin -0.8113 0.4691 -0.03 6.02 0 1.75 A4GALT 53947 alpha 1,4-galactosyltransferase 0.4383 0.7128 0.11 6.304 0 2.30 AACS 65985 acetoacetyl-CoA synthetase 0.3595 0.7664 0.03 3.534 0.00388 0.38 AADAC 13 arylacetamide deacetylase (esterase) 0.569 0.6216 0.16 0.005588 0.9968 0.00 AADAT 51166 aminoadipate aminotransferase -0.9577 0.3876 -0.11 0.8123 0.4752 0.24 AAK1 22848 AP2 associated kinase 1 -1.261 0.2505 -0.25 0.8232 0.4689 0.12 AAMP 14 angio-associated, migratory cell protein 0.873 0.4351 0.07 1.656 0.1476 0.06 AANAT 15 arylalkylamine N-acetyltransferase -0.3998 0.7394 -0.08 0.8486 0.456 0.18 AARS 16 alanyl-tRNA synthetase 5.517 0 0.34 8.616 0 0.69 AARS2 57505 alanyl-tRNA synthetase 2, mitochondrial (putative) 1.701 0.1158 0.35 0.5011 0.6622 0.07 AARSD1 80755 alanyl-tRNA synthetase domain containing 1 4.403 9.52E-05 0.52 1.279 0.2609 0.13 AASDH 132949 aminoadipate-semialdehyde dehydrogenase -0.8921 0.4247 -0.12 -2.564 0.02993 -0.32 AASDHPPT 60496 aminoadipate-semialdehyde