Plants, Tissues and Nutrition Plant Types and Their Evolution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transcript Profiling of a Novel Plant Meristem, the Monocot Cambium
Journal of Integrative JIPB Plant Biology Transcript profiling of a novel plant meristem, the monocot cambiumFA Matthew Zinkgraf1,2, Suzanne Gerttula1 and Andrew Groover1,3* 1. US Forest Service, Pacific Southwest Research Station, Davis, California, USA 2. Department of Computer Science, University of California, Davis, USA 3. Department of Plant Biology, University of California, Davis, USA Article *Correspondence: Andrew Groover ([email protected]) doi: 10.1111/jipb.12538 Abstract While monocots lack the ability to produce a xylem tissues of two forest tree species, Populus Research vascular cambium or woody growth, some monocot trichocarpa and Eucalyptus grandis. Monocot cambium lineages evolved a novel lateral meristem, the monocot transcript levels showed that there are extensive overlaps cambium, which supports secondary radial growth of between the regulation of monocot cambia and vascular stems. In contrast to the vascular cambium found in woody cambia. Candidate regulatory genes that vary between the angiosperm and gymnosperm species, the monocot monocot and vascular cambia were also identified, and cambium produces secondary vascular bundles, which included members of the KANADI and CLE families involved have an amphivasal organization of tracheids encircling a in polarity and cell-cell signaling, respectively. We suggest central strand of phloem. Currently there is no information that the monocot cambium may have evolved in part concerning the molecular genetic basis of the develop- through reactivation of genetic mechanisms involved in ment or evolution of the monocot cambium. Here we vascular cambium regulation. report high-quality transcriptomes for monocot cambium Edited by: Chun-Ming Liu, Institute of Crop Science, CAAS, China and early derivative tissues in two monocot genera, Yucca Received Feb. -

Tansley Review Evolution of Development of Vascular Cambia and Secondary Growth
New Phytologist Review Tansley review Evolution of development of vascular cambia and secondary growth Author for correspondence: Rachel Spicer1 and Andrew Groover2 Andrew Groover 1The Rowland Institute at Harvard, Cambridge, MA, USA; 2Institute of Forest Genetics, Pacific Tel: +1 530 759 1738 Email: [email protected] Southwest Research Station, USDA Forest Service, Davis, CA, USA Received: 29 December 2009 Accepted: 14 February 2010 Contents Summary 577 V. Evolution of development approaches for the study 587 of secondary vascular growth I. Introduction 577 VI. Conclusions 589 II. Generalized function of vascular cambia and their 578 developmental and evolutionary origins Acknowledgements 589 III. Variation in secondary vascular growth in angiosperms 581 References 589 IV. Genes and mechanisms regulating secondary vascular 584 growth and their evolutionary origins Summary New Phytologist (2010) 186: 577–592 Secondary growth from vascular cambia results in radial, woody growth of stems. doi: 10.1111/j.1469-8137.2010.03236.x The innovation of secondary vascular development during plant evolution allowed the production of novel plant forms ranging from massive forest trees to flexible, Key words: forest trees, genomics, Populus, woody lianas. We present examples of the extensive phylogenetic variation in sec- wood anatomy, wood formation. ondary vascular growth and discuss current knowledge of genes that regulate the development of vascular cambia and woody tissues. From these foundations, we propose strategies for genomics-based research in the evolution of development, which is a next logical step in the study of secondary growth. I. Introduction this pattern characterizes most extant forest trees, significant variation exists among taxa, ranging from extinct woody Secondary vascular growth provides a means of radially lycopods and horsetails with unifacial cambia (Cichan & thickening and strengthening plant axes initiated during Taylor, 1990; Willis & McElwain, 2002), to angiosperms primary, or apical growth. -

Chapter 5: the Shoot System I: the Stem
Chapter 5 The Shoot System I: The Stem THE FUNCTIONS AND ORGANIZATION OF THE SHOOT SYSTEM PRIMARY GROWTH AND STEM ANATOMY Primary Tissues of Dicot Stems Develop from the Primary Meristems The Distribution of the Primary Vascular Bundles Depends on the Position of Leaves Primary Growth Differs in Monocot and Dicot Stems SECONDARY GROWTH AND THE ANATOMY OF WOOD Secondary Xylem and Phloem Develop from Vascular Cambium Wood Is Composed of Secondary Xylem Gymnosperm Wood Differs from Angiosperm Wood Bark Is Composed of Secondary Phloem and Periderm Buds Are Compressed Branches Waiting to Elongate Some Monocot Stems Have Secondary Growth STEM MODIFICATIONS FOR SPECIAL FUNCTIONS THE ECONOMIC VALUE OF WOODY STEMS SUMMARY ECONOMIC BOTANY: How Do You Make A Barrel? 1 KEY CONCEPTS 1. The shoot system is composed of the stem and its lateral appendages: leaves, buds, and flowers. Leaves are arranged in different patterns (phyllotaxis): alternate, opposite, whorled, and spiral. 2. Stems provide support to the leaves, buds, and flowers. They conduct water and nutrients and produce new cells in meristems (shoot apical meristem, primary and secondary meristems). 3. Dicot stems and monocot stems are usually different. Dicot stems tend to have vascular bundles distributed in a ring, whereas in monocot stems they tend to be scattered. 4. Stems are composed of the following: epidermis, cortex and pith, xylem and phloem, and periderm. 5. Secondary xylem is formed by the division of cells in the vascular cambium and is called wood. The bark is composed of all of the tissues outside the vascular cambium, including the periderm (formed from cork cambium) and the secondary phloem. -

Development and Cell Cycle Activity of the Root Apical Meristem in the Fern Ceratopteris Richardii
G C A T T A C G G C A T genes Article Development and Cell Cycle Activity of the Root Apical Meristem in the Fern Ceratopteris richardii Alejandro Aragón-Raygoza 1,2 , Alejandra Vasco 3, Ikram Blilou 4, Luis Herrera-Estrella 2,5 and Alfredo Cruz-Ramírez 1,* 1 Molecular and Developmental Complexity Group at Unidad de Genómica Avanzada, Laboratorio Nacional de Genómica para la Biodiversidad, Cinvestav Sede Irapuato, Km. 9.6 Libramiento Norte Carretera, Irapuato-León, Irapuato 36821, Guanajuato, Mexico; [email protected] 2 Metabolic Engineering Group, Unidad de Genómica Avanzada, Laboratorio Nacional de Genómica para la Biodiversidad, Cinvestav Sede Irapuato, Km. 9.6 Libramiento Norte Carretera, Irapuato-León, Irapuato 36821, Guanajuato, Mexico; [email protected] 3 Botanical Research Institute of Texas (BRIT), Fort Worth, TX 76107-3400, USA; [email protected] 4 Laboratory of Plant Cell and Developmental Biology, Division of Biological and Environmental Sciences and Engineering (BESE), King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Saudi Arabia; [email protected] 5 Institute of Genomics for Crop Abiotic Stress Tolerance, Department of Plant and Soil Science, Texas Tech University, Lubbock, TX 79409, USA * Correspondence: [email protected] Received: 27 October 2020; Accepted: 26 November 2020; Published: 4 December 2020 Abstract: Ferns are a representative clade in plant evolution although underestimated in the genomic era. Ceratopteris richardii is an emergent model for developmental processes in ferns, yet a complete scheme of the different growth stages is necessary. Here, we present a developmental analysis, at the tissue and cellular levels, of the first shoot-borne root of Ceratopteris. -
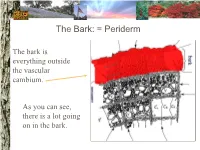
The Bark: = Periderm
The Bark: = Periderm The bark is everything outside the vascular cambium. As you can see, there is a lot going on in the bark. The Bark: periderm: phellogen (cork cambium): The phellogen is the region of cell division that forms the periderm tissues. Phellogen development influences bark appearance. The Bark: periderm: phellem (cork): Phellem replaces the epidermis as the tree increases in girth. Photosynthesis can take place in some trees both through the phellem and in fissures. The Bark: periderm: phelloderm: Phelloderm is active parenchyma tissue. Parenchyma cells can be used for storage, photosynthesis, defense, and even cell division! The Bark: phloem: Phloem tissue makes up the inner bark. However, it is vascular tissue formed from the vascular cambium. The Bark: phloem: sieve tube elements: Sieve tube elements actively transport photosynthates down the stem. Conifers have sieve cells instead. The cambium: The cambium is the primary meristem producing radial growth. It forms the phloem & xylem. The Xylem (wood): The xylem includes everything inside the vascular cambium. The Xylem: a growth increment (ring): The rings seen in many trees represent one growth increment. Growth rings provide the texture seen in wood. The Xylem: vessel elements: Hardwood species have vessel elements in addition to trachieds. Notice their location in the growth rings of this tree The Xylem: fibers: Fibers are cells with heavily lignified walls making them stiff. Many fibers in sapwood are alive at maturity and can be used for storage. The Xylem: axial parenchyma: Axial parenchyma is living tissue! Remember that parenchyma cells can be used for storage and cell division. -

Tree Anatomy Stems and Branches
Tree Anatomy Series WSFNR14-13 Nov. 2014 COMPONENTSCOMPONENTS OFOF PERIDERMPERIDERM by Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia Around tree roots, stems and branches is a complex tissue. This exterior tissue is the environmental face of a tree open to all sorts of site vulgarities. This most exterior of tissue provides trees with a measure of protection from a dry, oxidative, heat and cold extreme, sunlight drenched, injury ridden site. The exterior of a tree is both an ecological super highway and battle ground – comfort and terror. This exterior is unique in its attributes, development, and regeneration. Generically, this tissue surrounding a tree stem, branch and root is loosely called bark. The tissues of a tree, outside or more exterior to the xylem-containing core, are varied and complexly interwoven in a relatively small space. People tend to see and appreciate the volume and physical structure of tree wood and dismiss the remainder of stem, branch and root. In reality, tree life is focused within these more exterior thin tissue sets. Outside of the cambium are tissues which include transport cells, structural support cells, generation cells, and cells positioned to help, protect, and sustain other cells. All of this life is smeared over the circumference of a predominately dead physical structure. Outer Skin Periderm (jargon and antiquated term = bark) is the most external of tree tissues providing protection, water conservation, insulation, and environmental sensing. Periderm is a protective tissue generated over and beyond live conducting and non-conducting cells of the food transport system (phloem). -

Genetic Suppression of Plant Development and Chloroplast Biogenesis Via the Snowy Cotyledon 3 and Phytochrome B Pathways
CSIRO PUBLISHING Functional Plant Biology, 2015, 42, 676–686 http://dx.doi.org/10.1071/FP15026 Genetic suppression of plant development and chloroplast biogenesis via the Snowy Cotyledon 3 and Phytochrome B pathways Diep Ganguly A, Peter Crisp A, Klaus Harter B, Barry J. Pogson A and Verónica Albrecht-Borth A,C AARC (Australian Research Council) Centre of Excellence in Plant Energy Biology, Research School of Biology, Australian National University Canberra, Acton, ACT 0200, Australia. BZentrum für Molekularbiologie der Pflanzen, Plant Physiology, University of Tübingen, 72076 Tübingen, Germany. CCorresponding author. Email: [email protected] Abstract. Plant development is regulated by external and internal factors such as light and chloroplast development. A revertant of the Arabidopsis thaliana (L.) Heyhn. chloroplast biogenesis mutant snowy cotyledon 3 (sco3–1) was isolated partially recovering the impaired chloroplast phenotype. The mutation was identified in the Phytochrome B (PhyB) gene and is a result of an amino acid change within the PAS repeat domain required for light-induced nuclear localisation. An independent phyB-9 mutation was crossed into sco3–1 mutants, resulting in the same partial reversion of sco3–1. Further analysis demonstrated that SCO3 and PhyB influence the greening process of seedlings and rosette leaves, embryogenesis, rosette formation and flowering. Interestingly, the functions of these proteins are interwoven in various ways, suggesting a complex genetic interaction. Whole-transcriptome profiling of sco3–1phyB-9 indicated that a completely distinct set of genes was differentially regulated in the double mutant compared with the single sco3–1 or phyB-9 mutants. Thus, we hypothesise that PhyB and SCO3 genetically suppress each other in plant and chloroplast development. -

BIO 102 General Biology Lecture Outline Plantae I. Introduction A. Taxonomy Domain
BIO 102 General Biology Lecture Outline Plantae I. Introduction A. Taxonomy Domain: Eukarya Kingdom: Plantae B. General characteristics Multicellular Autotrophic / Photosynthetic Cellulose cell wall Reproduction – alternation of generations C. Life cycle Alternation of generations Haploid = gametophyte, which produces gametes via mitosis Diploid = sporophyte, which produces spores via meiosis Meiosis = cell division from 2n to n Mitosis = cell division either from 2n to 2n, OR from n to n Fertilization = combine gametes: n + n = 2n Homospory vs. heterospory II. Major Groups of Plants A. Characterized by 4 criteria in branching manner Vascular vs. non-vascular Seed production or lack thereof Types of seeds produced Development & morphology B. Non-tracheophytes (nonvascular plants) General characteristics No vascular tissue Gametophyte (haploid) generation dominant Requires water for reproduction Examples Liverworts Hornworts Mosses Moss reproductive cycle C. Tracheophytes General characteristics Vascular tissue: Xylem and Phloem Specialized tissues: Leaves Roots Stems Cuticles Stomata Sporophyte (diploid) generation dominant Divisions based on seed-bearing vs. non-seed bearing (Seed vs. spore) D. Seedless plants BIO 102 General Biology Lecture Outline General characteristics Have vascular tissue but lack seeds Examples Ferns Whisk ferns Club mosses Horsetails E. Seed-bearing plants General characteristics Have vascular tissue and seeds Divisions based on whether seeds are “covered” or not F. Gymnosperms General characteristics Vascular plants with “naked” seeds = i.e., no fruit Examples Conifers Gingkos Cycads Gnetophytes Gymnosperm life cycle G. Angiosperms General characteristics Vascular plants with “covered” seeds = i.e., seeds within fruits Fruits are derived from flowers Angiosperm = flowering plant Divisions are based on development and plant morphology (i.e., structures) Angiosperm life cycle H. -

Dicot/Monocot Root Anatomy the Figure Shown Below Is a Cross Section of the Herbaceous Dicot Root Ranunculus. the Vascular Tissu
Dicot/Monocot Root Anatomy The figure shown below is a cross section of the herbaceous dicot root Ranunculus. The vascular tissue is in the very center of the root. The ground tissue surrounding the vascular cylinder is the cortex. An epidermis surrounds the entire root. The central region of vascular tissue is termed the vascular cylinder. Note that the innermost layer of the cortex is stained red. This layer is the endodermis. The endodermis was derived from the ground meristem and is properly part of the cortex. All the tissues inside the endodermis were derived from procambium. Xylem fills the very middle of the vascular cylinder and its boundary is marked by ridges and valleys. The valleys are filled with phloem, and there are as many strands of phloem as there are ridges of the xylem. Note that each phloem strand has one enormous sieve tube member. Outside of this cylinder of xylem and phloem, located immediately below the endodermis, is a region of cells called the pericycle. These cells give rise to lateral roots and are also important in secondary growth. Label the tissue layers in the following figure of the cross section of a mature Ranunculus root below. 1 The figure shown below is that of the monocot Zea mays (corn). Note the differences between this and the dicot root shown above. 2 Note the sclerenchymized endodermis and epidermis. In some monocot roots the hypodermis (exodermis) is also heavily sclerenchymized. There are numerous xylem points rather than the 3-5 (occasionally up to 7) generally found in the dicot root. -

Anatomical Traits Related to Stress in High Density Populations of Typha Angustifolia L
http://dx.doi.org/10.1590/1519-6984.09715 Original Article Anatomical traits related to stress in high density populations of Typha angustifolia L. (Typhaceae) F. F. Corrêaa*, M. P. Pereiraa, R. H. Madailb, B. R. Santosc, S. Barbosac, E. M. Castroa and F. J. Pereiraa aPrograma de Pós-graduação em Botânica Aplicada, Departamento de Biologia, Universidade Federal de Lavras – UFLA, Campus Universitário, CEP 37200-000, Lavras, MG, Brazil bInstituto Federal de Educação, Ciência e Tecnologia do Sul de Minas Gerais – IFSULDEMINAS, Campus Poços de Caldas, Avenida Dirce Pereira Rosa, 300, CEP 37713-100, Poços de Caldas, MG, Brazil cInstituto de Ciências da Natureza, Universidade Federal de Alfenas – UNIFAL, Rua Gabriel Monteiro da Silva, 700, CEP 37130-000, Alfenas, MG, Brazil *e-mail: [email protected] Received: June 26, 2015 – Accepted: November 9, 2015 – Distributed: February 28, 2017 (With 3 figures) Abstract Some macrophytes species show a high growth potential, colonizing large areas on aquatic environments. Cattail (Typha angustifolia L.) uncontrolled growth causes several problems to human activities and local biodiversity, but this also may lead to competition and further problems for this species itself. Thus, the objective of this study was to investigate anatomical modifications on T. angustifolia plants from different population densities, once it can help to understand its biology. Roots and leaves were collected from natural populations growing under high and low densities. These plant materials were fixed and submitted to usual plant microtechnique procedures. Slides were observed and photographed under light microscopy and images were analyzed in the UTHSCSA-Imagetool software. The experimental design was completely randomized with two treatments and ten replicates, data were submitted to one-way ANOVA and Scott-Knott test at p<0.05. -

Eudicots Monocots Stems Embryos Roots Leaf Venation Pollen Flowers
Monocots Eudicots Embryos One cotyledon Two cotyledons Leaf venation Veins Veins usually parallel usually netlike Stems Vascular tissue Vascular tissue scattered usually arranged in ring Roots Root system usually Taproot (main root) fibrous (no main root) usually present Pollen Pollen grain with Pollen grain with one opening three openings Flowers Floral organs usually Floral organs usually in in multiples of three multiples of four or five © 2014 Pearson Education, Inc. 1 Reproductive shoot (flower) Apical bud Node Internode Apical bud Shoot Vegetative shoot system Blade Leaf Petiole Axillary bud Stem Taproot Lateral Root (branch) system roots © 2014 Pearson Education, Inc. 2 © 2014 Pearson Education, Inc. 3 Storage roots Pneumatophores “Strangling” aerial roots © 2014 Pearson Education, Inc. 4 Stolon Rhizome Root Rhizomes Stolons Tubers © 2014 Pearson Education, Inc. 5 Spines Tendrils Storage leaves Stem Reproductive leaves Storage leaves © 2014 Pearson Education, Inc. 6 Dermal tissue Ground tissue Vascular tissue © 2014 Pearson Education, Inc. 7 Parenchyma cells with chloroplasts (in Elodea leaf) 60 µm (LM) © 2014 Pearson Education, Inc. 8 Collenchyma cells (in Helianthus stem) (LM) 5 µm © 2014 Pearson Education, Inc. 9 5 µm Sclereid cells (in pear) (LM) 25 µm Cell wall Fiber cells (cross section from ash tree) (LM) © 2014 Pearson Education, Inc. 10 Vessel Tracheids 100 µm Pits Tracheids and vessels (colorized SEM) Perforation plate Vessel element Vessel elements, with perforated end walls Tracheids © 2014 Pearson Education, Inc. 11 Sieve-tube elements: 3 µm longitudinal view (LM) Sieve plate Sieve-tube element (left) and companion cell: Companion cross section (TEM) cells Sieve-tube elements Plasmodesma Sieve plate 30 µm Nucleus of companion cell 15 µm Sieve-tube elements: longitudinal view Sieve plate with pores (LM) © 2014 Pearson Education, Inc. -

Immunoprofiling of Rice Root Cortex Reveals Two Cortical Subdomains
METHODS published: 07 January 2016 doi: 10.3389/fpls.2015.01139 Immunoprofiling of Rice Root Cortex Reveals Two Cortical Subdomains Sophia Henry, Fanchon Divol, Mathilde Bettembourg, Charlotte Bureau, Emmanuel Guiderdoni, Christophe Périn * and Anne Diévart * CIRAD, UMR AGAP, Montpellier, France The formation and differentiation of aerenchyma, i.e., air-containing cavities that are critical for flooding tolerance, take place exclusively in the cortex. The understanding of development and differentiation of the cortex is thus an important issue; however, studies on this tissue are limited, partly because of the lack of available molecular tools. We screened a commercially available library of cell wall antibodies to identify markers of cortical tissue in rice roots. Out of the 174 antibodies screened, eight were cortex-specific. Our analysis revealed that two types of cortical tissues are present in rice root seedlings. We named these cell layers “inner” and “outer” based on their location relative to the stele. We then used the antibodies to clarify cell identity in lateral roots. Without these markers, previous studies could not distinguish between the cortex and sclerenchyma in small lateral roots. By immunostaining lateral root sections, we showed that the internal ground tissue in small lateral roots has outer cortical identity. Edited by: Elison B. Blancaflor, Keywords: rice root, cortex, markers, antibodies, lateral roots, tissue identity, confocal microscopy, confocal The Samuel Roberts Noble imaging Foundation, USA Reviewed by: David Domozych, INTRODUCTION Skidmore College, USA Laura Elizabeth Bartley, Rice has a complex root architecture with a mix of embryonic and post-embryonic roots. The University of Oklahoma, USA radicle emerges first during germination, followed soon thereafter by embryonic coronary roots *Correspondence: (Rebouillat et al., 2009; Coudert et al., 2010).