The Cr(VI) Stability in Contaminated Coastal Groundwater: Salinity As a Driving Force
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sterea Ellada / Griechenland
tourismus Hamburg ist schön – aber waren Sie schon Wichtigkeit für die Gemeinschaft (SCI) Wildschweine und Füchse, vereinzelt auch heiß und trocken. Die jährlichen Niederschlä- und 202 spezielle Schutzzonen (SPA) auf. Wölfe und Bären. Die Fauna der Inseln ge schwanken zwischen 400 und 1000 mm. mal in Griechenland? Von den 5500–6000 Pflanzenarten und unterscheidet sich wesentlich von der des Da Griechenland sehr gebirgig ist, ist Winter- Unterarten in Griechenland sind 20 Pro- Festlandes, regional findet sich dort z.B. sport durchaus möglich, es existieren 19 Win- zent endemisch, die hohe Anzahl an Arten die Karettschildkröte auf Zakynthos, tersportgebiete unterschiedlicher Größe. Ein Griechenland liegt am östlichen Mittel- Größere Flüsse, die ganzjährig Wasser Flora und Fauna sind kulturell eng mit beinhaltet auch einen überdurchschnitt- sowie als eine der ende- kleiner Teil im Nordwesten des Fest- meer in Südeuropa und setzt sich geo- führen, gibt es überwiegend im Norden antiken Mythen verbunden, seltener lich hohen Anteil an Heilpflanzen. Sie mischen Arten die landes liegt in der graphisch aus dem griechischen Festland des griechischen Festlandes, die dort zur jedoch mit den Hauptgottheiten. Chloris bildeten einst die Grundlage für eine gemäßigten am südlichen Ende des Balkans, der Bewässerung der fruchtbaren Täler und war die Göttin der Blumen, die für Hera ausgeprägte Volksmedizin, aber auch für Wildziege auf Kreta. Klimazone. Halbinsel Peloponnes, die jedoch durch zum geringen Anteil der Energiegewin- die Pflanzen sprießen ließ, Nymphen die Asklepiaden. Den Großteil der Vegeta- Im Schmetterlingstal auf Rhodos ist die den Bau des Kanals von Korinth (einge- nung genutzt werden. Darunter der Pinios, waren für das Leben der Pflanzen verant- tion machen immergrüne Pflanzen (breit- seltene Schmetterlingsart Panaxia quadri- weiht 1893) vom Festland getrennt Axios, Strymonas, Nestos und Evros. -

Dirfys-Messapia FY R O M
Montenegro Bulgaria Dirfys-Messapia FY R O M Activation ID: EMSN-025 Albania Glide Number: N/A P roduct N.: 04DIR FY S, v1, English Legend Greece Hazard Level Points of Interest Transportation Hazard Level Fire Ex tent Greece LU/LC (sqkm) Turkey Dirfys - Greece Null IC H ospital Secondary V ery Low Very Low Low Medium High Very High Fire station Tertiary Soil Erosion Hazard Zones Low Places ×Ñ Medium !. Education Local and Service Detail Tile 4009 V illage IH Olive groves - 0,089 0,104 0,098 - H igh Track !. Toponym V ery H igh (!S Sports P roduction date: 27/7/2016 Bridge & overpass Pastures - Úð Industrial facilities - 0,022 - - Greece Mitigation Measures Utility network Buildings Electricity infrastructure !( ô!E Cartographic Information Mulch treatment H igh voltage grid Commercial, P ublic & Coniferous forest 0,213 0,476 0,339 0,006 - !n Dam P rivate Services 4009 Full color A1, high resolution (300dpi) 1:5.000 Physiography Hydrography Industry & U tilities Sclerophyllous vegetation - 0,069 0,008 - - 300 P rimary R iver P lace of worship 0 0,05 0,1 0,2 0,3 0,4 Secondary Stream Transitional woodland/shrub - 0,067 0,140 - - U nclassified Km # ± H eight spots Coastline Areas affected by fire - 0,047 0,709 0,287 0,309 Grid: GGR S87 (H ellenic Geodetic R eference System 1987) Tick marks: W GS 84 geographical coordinate system 476500 477000 477500 478000 478500 479000 23°44'0"E 23°44'30"E 23°45'0"E 23°45'30"E 0 0 0 0 5 5 6 6 7 7 2 2 4 4 N " N " 0 4 ' 0 0 0 ' 8 8 3 00 ° 5 5 3 ° 8 00 8 3 3 0 0 0 0 0 0 6 6 7 7 2 2 4 524m 4 0 0 5 400 M e -

The August 9, 2020 Evia (Central Greece) Flood
ISSN 2653-9454 Issue No.19 | August 2020 The August 9, 2020 Evia (Central Greece) Flood Prof. Efthymis Lekkas PhD c Nafsika-Ioanna Spyrou PhD c Evelina Kotsi PhD c Christos Filis Dr. Michalis Diakakis Prof. Contantinos Cartalis Ass. Prof. Emmanuel Vassilakis MSc Thalia Mavrakou BEng Panagiotis Sartabakos PhD c Marilia Gogou PhD c Kat.-Navs. Katsetsiadou MSc Vanessa Barsaki Dr. Kostis Lagouvardos Dr. Vassiliki Kotroni Dr. Athanasios Karagiannidis MSc Stavros Dafis Prof. Isaak Parcharidis MSc Andreas Karavias MSc Despoina Bafi MSc Ioannis Gougoustamos ISSN 2653-9454 Issue No. 19, August 2020 | 2 About Non-periodic publication of the Post-graduate Studies Publishers: Program “Environmental Disasters & Crises Dr. Efthymis Lekkas Management Strategies" of the National & Kapodistrian Dr. Nikolaos Voulgaris University of Athens, issued after significant events for Dr. Stylianos Lozios the immediate information of the scientific community and the general public. The publication includes also Technical Editing: scientific data from various research teams from PhD c. Spyridon Mavroulis universities, organizations and research institutes. Communication: PhD c. Spyridon Mavroulis ([email protected]) MSc Alexia Grambas ([email protected]) MSc Katerina-Nafsika Katsetsiadou ([email protected]) Scientific Mission Copyrights Of the National and Kapodistrian University of Athens, Faculty of Geology All copyrights of scientific data belong to their and Geoenvironment, Department of Dynamic Tectonic Applied Geology respective owners, while the copyrights of this publication belong to the publishers. Contributors Dr. Efthymis Lekkas, Professor of Dynamic, Tectonic & Applied Geology & Natural Disaster Management Dr. Konstantinos Lagouvardos, Director of Research IERSD/National Observatory of Athens Cited as Dr. Constantinos Cartalis, Professor of Environmental Physics at the University of Athens Lekkas, E., Spyrou N-I., Kotsi E., Filis, Ch., Diakakis, M., Dr. -

University Microfilms International
ANCIENT EUBOEA: STUDIES IN THE HISTORY OF A GREEK ISLAND FROM EARLIEST TIMES TO 404 B.C. Item Type text; Dissertation-Reproduction (electronic) Authors Vedder, Richard Glen, 1950- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 11/10/2021 05:15:39 Link to Item http://hdl.handle.net/10150/290465 INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. You will find a good image of the page in the adjacent frame. -

Spatial Diversity of Cr Distribution in Soil and Groundwater Sites in Relation with Land Use Management in a Mediterranean Region: the Case of C
Science of the Total Environment 651 (2019) 656–667 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Spatial diversity of Cr distribution in soil and groundwater sites in relation with land use management in a Mediterranean region: The case of C. Evia and Assopos-Thiva Basins, Greece Ifigeneia Megremi, Charalampos Vasilatos ⁎, Emmanuel Vassilakis, Maria Economou-Eliopoulos Department of Geology and Geoenvironment, University of Athens, Athens 15784, Greece HIGHLIGHTS GRAPHICAL ABSTRACT • Spatial distribution of Cr and other ele- ments in an area of Greece investigated • Maps of land use and element contents in soil and groundwater were devel- oped. • GIS and multivariate statistics were ap- plied to assess the origin of Cr contami- nation. • Maps help distinguish Cr of geogenic and anthropogenic origin and saliniza- tion. • These provide information for sustain- able land management. article info abstract Article history: The present study compiles new and literature data in a GIS platform aiming to (a) evaluate the extent and mag- Received 4 July 2018 nitude of Cr contamination in a Mediterranean region (Assopos-Thiva and Central Evia (Euboea) Basins, Greece); Received in revised form 14 September 2018 (b) combine spatial distribution of Cr in soil and groundwater with land use maps; (c) determine geochemical Accepted 14 September 2018 constraints on contamination by Cr; and (d) provide information that will be useful for better management of Available online 15 September 2018 land use in a Mediterranean type ecosystem in order to prevent further degradation of natural resources. Editor: Mae Mae Sexauer Gustin The spatial diversity of Cr distribution in soils and groundwater throughout the C. -

Central Evia Island, Greece)
geosciences Article Geomorphic Evolution of the Lilas River Fan Delta (Central Evia Island, Greece) Efthimios Karymbalis 1,* , Kanella Valkanou 1, Ioannis Tsodoulos 1,2, George Iliopoulos 3 , Konstantinos Tsanakas 1, Vasilis Batzakis 1, Giorgos Tsironis 1, Christina Gallousi 1, Konstantinos Stamoulis 2 and Konstantinos Ioannides 2 1 Department of Geography, Harokopio University, 70 El. Venizelou Str., GR-17671 Athens, Greece; [email protected] (K.V.); [email protected] (I.T.); [email protected] (K.T.); [email protected] (V.B.); [email protected] (G.T.); [email protected] (C.G.) 2 Department of Physics, University of Ioannina, GR-45110 Ioannina, Greece; [email protected] (K.S.); [email protected] (K.I.) 3 Department of Geology, University of Patras, GR-26504 Rio Achaia, Greece; [email protected] * Correspondence: [email protected]; Tel.: +30-210-954-9159 Received: 26 August 2018; Accepted: 25 September 2018; Published: 26 September 2018 Abstract: This paper presents the results of geomorphological investigations carried out on the Lilas River fan delta in central Evia Isl., Greece. A geomorphological map has been prepared using Digital Elevation Model analysis, aerial photos and Google Earth image interpretation, a reliable map of 1846, and extensive fieldwork. The Holocene sequence stratigraphy of the fan delta has been studied based on profiles of seven deep cores drilled by the municipal authorities. Two additional shallow boreholes were drilled with a portable drilling set and collected samples were analyzed using micropaleontological and grain size analysis methods while four sediment samples were dated using optically stimulated luminescence (OSL) techniques. During the early Holocene, most of the fan delta plain was a shallow marine environment. -

Goldschmidt-2019 Argyrakietal
GROUNDWATER PRESSURES IN Cr(VI) IMPACTED AQUIFERS OF CENTRAL GREECE Ariadne Argyraki1 ([email protected]), Konstantina Pyrgaki1, Efstratios Kelepertzis1, Ifigeneia Megremi1, Fotini Botsou1, Maria Hatzaki1, Dimitris Dermatas2 1 National and Kapodistrian University of Athens, Panepistimiopolis Zographou, 15784 Athens, Greece 2 National Technical University of Athens, Athens 15780, Greece INTRODUCTION LAND USES & WATER DEMANDS Annual Water Demand of Loutraki- Annual Demand of Oinofyta Groundwater quantity and quality can be directly affected through changes in precipitation, PROPORTION OF LANDUSE TYPE IN THE STUDY AREAS Perachora Municipality (106 m3 ) (Asopos) Municipality (106 m3) (NUMBERS INDICATE AREA IN SQ. KM) evapotranspiration, recharge rates, and indirectly through changes in land use, irrigation and 1% 14% other human activities. Here we focus on the water quality of four groundwater bodies in 14% 45% 54% 5% central Greece. A common feature of the studied aquifers is the presence of geogenic Cr(VI), 350 67% linked to ophiolithic rock occurrences. Our data are interpreted within the frame of the ERANETMED CrITERIA project. 548 62 60 Irrigation Drinking Livestock Irrigation Drinking Livestock Industrial In CrITERIA, groundwater quality is assessed following a gradient from relatively wet to dry 406 Annual Demand of Thiva Average Annual Water Demand of conditions along the Tethyan Suture Zone that structurally defines locations of ophiolite Municipality (106 m3) Dirfys-Messapia Municipality (Evia) 22 1 6 3 occurrences, extending from the eastern Mediterranean to Oman. 13 106 6 (10 m ) 5 4% 49 4 5% 9% THIVA- ASOPOS EVIA LOUTRAKI SCHINOS 8% AIM Urban Industrial/ Mineral Extraction 91% 81% Irrigated agricultural Non-irrigated/ Forest/ Bare rocks Irrigation Drinking Livestock Industrial Irrigation Drinking Livestock Industrial We examine the linkage between the presence of mainly geogenic Cr(VI) in groundwater Estimated water demands in the study areas are driven by the current state of landuse. -
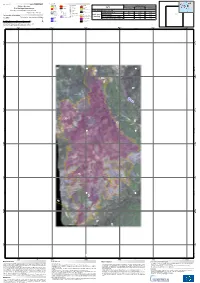
Dirfys-Messapia FY R O M
Montenegro Bulgaria Dirfys-Messapia FY R O M Activation ID: EMSN-025 Albania Glide N umber: N /A P roduct N.: 04DIR FY S, v1, English Legend Damage L evel Points of Interest Transportation Greece Greece Fire Ex tent Damage Level Turkey Dirfys - Greece Not burnt IC H ospital Secondary P artly Burnt Dyrfis No Data Ñ× Fire station Tertiary Fire Damage Assessment H eavily burnt Not burnt Partly burnt Heavily burnt Local and Service Delineation and Grading Map - Detail Tile 4009 IH Education Physiography Places Track Total area affected: 133,7 ha !(S Sports P roduction date: 27/7/2016 300 P rimary !. V illage Bridge & overpass Coniferous forest 0,8 31,0 65,8 Úð Industrial facilities Secondary !. Toponym Greece Buildings # H eight spots !ôE Electricity infrastructure Land Cover/ Olive groves 2,5 13,8 11,7 Cartographic Information Utility network Commercial, P ublic & !( H igh voltage grid !n Dam P rivate Services Use (ha) Sclerophyllous vegetation - 1,4 1,4 4009 1:5.000 Full color A1, low resolution (100dpi) Hydrography R iver Industry & U tilities Transitional woodland/shrub - 1,5 3,9 Stream P lace of worship 0 0,05 0,1 0,2 0,3 0,4 Coastline Km ± U nclassified Grid:GGR S87 (H ellenic Geodetic R eference System 1987) Tick marks: W GS 84 geographical coordinate system 476500 477000 477500 478000 478500 479000 23°44'0"E 23°44'30"E 23°45'0"E 23°45'30"E 0 0 0 0 5 5 6 6 7 7 2 2 4 4 N " N " 0 4 ' 0 0 0 ' 8 8 3 00 ° 5 5 3 ° 8 00 8 3 3 0 0 0 0 0 0 6 6 7 7 2 2 4 524m 4 0 0 5 400 M e sa p io s 0 0 0 0 5 5 5 5 7 7 2 2 4 4 400 0 0 3 479m N " N " 0 0 3 -
Sarakenos Cave in Boeotia, from Palaeo Lithic to The
Eura sian Pre history, 6 (1–2): 199–231. SARAKENOS CAVE IN BOEOTIA, FROM PALAEOLITHIC TO THE EARLY BRONZE AGE Adamantios Sampson1, Janusz K. Koz³owski2, Ma³gorzata Kaczanowska3, Anna Budek4, Adam Nadachowski5, 6, Teresa Tomek6 and Barbara Miêkina6 1 Aegean Uni ver sity Rhodes, De part ment of Med i ter ra nean Ar chae ol ogy, Demokratios Ave, Rhodes, Greece 2 In sti tute of Ar chae ol ogy, Jagiellonian Uni ver sity, ul. Go³êbia 11, 31-007 Kraków, Po land, [email protected] 3 Ar chae o log i cal Mu seum, ul. Senacka 3, Kraków, Po land 4 In sti tute of Geography and Spa tial Organization, Pol ish Acad emy of Sci ence, ul. Œw. Jana 22, 31-016 Kraków, Po land 5 Departament of Palaeozoology, Zoo log i cal In sti tute, Wroc³aw Uni ver sity, Sienkiewicza 21, 50-355 Wroc³aw, Po land 6 In sti tute of System at ics and Evo lu tion of An i mals, Pol ish Acad emy of Sci ences, ul. S³awkowska 17, 31-016 Kraków, Po land Ab stract Sarakenos Cave, oc cupied since the Late Palaeolithic, is of crucial im portance for the study of the Mesolithic/Neo- lithic inter face. There is a striking contrast between the isolated Mesolithic, partic u larly Late Mesolithic occu pa tions with flake technol ogy adapted to the lo cal bad quality raw mate ri als, and with subsis tence economy based on fowling and plant gather ing, also adapted to the lo cal envi ron men tal condi tions, and the Early Neolithic groups arriv ing some 120–140 ra- diocar bon years later, with im ported raw mate ri als, macroblade technol ogy and ani mal breeding. -

The Settlements of the Middle and Late Byzantine Period in Euboea
The settlements of the Middle and Late Byzantine period in Euboea Pari KALAMARA Abstract Η διερεύνηση του θέματος των οικισμών της μέσης και ύστερης βυζαντινής περιόδου στην Εύβοια (από τα μέσα του 7ου αιώνα έως και το 1470: οθωμανική κατάληψη) ξεκίνησε από τη διαπίστωση ότι τα σωζόμενα στο πεδίο σαφώς οικιστικού χαρακτήρα κατάλοιπα είναι περιορισμένα (κυρίως πύργοι) και μεταξύ τους απομακρυσμένα, ενώ απουσιάζουν σχετικές συνθετικές μελέτες για την Εύβοια που συνεκτιμούν τεκμηριωμένα αρχαιολογικά δεδομένα και γραπτές πηγές. Η χωροθέτηση των οικιστι- κών πυρήνων, ο χρόνος δημιουργίας τους, η ταυτότητά τους (πόλεις-κάστρα, οχυρωμένοι οικισμοί, ατείχιστοι αγροτικοί οικισμοί κ.λπ.) καθώς και οι μεταξύ τους σχέσεις θεωρούνται ιδιαίτερα σημαντικά για την κατανόηση της ιστορίας του νησιού και του ρόλου του κατά τη μεσαιωνική περίοδο, λόγω και της σημασίας που το δίκτυο των πόλεων και των οικισμών είχε διαχρονικά στο Βυζάντιο για τον καθορισμό της επικράτειάς του και τον έλεγχο αυτής πολιτικά, στρατιωτικά και οικονομικά. Συνοψί- ζοντας τα δεδομένα παρατηρούνται τα ακόλουθα: Οι οικισμοί, πόλεις-κάστρα, οχυρωμένα πολίσματα και «χωρία», εντοπίζονται κατεξοχήν στις εύφορες περιοχές, σε παράλιες θέσεις που εξυπηρετούν και τη δια θαλάσσης επικοινωνία, όπως είναι η Κάρυστος, το Αλιβέρι, η Βάθεια κ.λπ., ή σε πεδιάδες ή οροπέδια της ενδοχώρας, όπως στις περιπτώσεις της περιοχής του Λίλλαντα ή της Δίρφυς, αλλά και στη ζώνη Αυλωναρίου – Κύμης. Εμφανής είναι, με βάση βέβαια τα έως σήμερα δεδομένα της έρευνας, η πυκνότητα των οικισμών στην κεντρική και νότια Εύβοια. Συνολικά σημειώνονται 25 οχυρωμένοι οικισμοί. Εξ αυτών, περίπου οι μισοί εμφανίζονται για πρώτη φορά στην ύστερη βυζαντινή περίοδο, όπως τα κάστρα Κλεισούρας, Μαντούκο, Argalia, Γιάλ- τρων (Β. -

Geomorpology 2006 Nileas Graben
Geomorphology 76 (2006) 363–374 www.elsevier.com/locate/geomorph Transverse fault zones of subtle geomorphic signature in northern Evia island (central Greece extensional province): An introduction to the Quaternary Nileas graben ⁎ Nikolaos Palyvos a, , Ioannis Bantekas b, Haralambos Kranis b a Istituto Nazionale di Geofisica e Vulcanologia, Via di Vigna murata 605, 00143 Rome, Italy b Faculty of Geology, Dynamic, Tectonic and Applied Geology Department, Panepistimioupolis Zografou, 157 84, Athens, Greece Received 11 March 2004; received in revised form 29 September 2005; accepted 8 December 2005 Available online 20 January 2006 Abstract Reconnaissance level geomorphological observations in the northern part of Evia (Euboea) Island, suggest that a major topographic feature, the 17 km long and 15 km wide Nileas depression (NDpr), corresponds to a previously undetected graben structure, bounded by fault zones of ENE–WSW to NE–SW general strike. These fault zones have been active in the Quaternary, since they affect the Neogene deposits of the Limni–Histiaia basin. They strike transverse to the NW–SE active fault zones that bound northern Evia in the specific area and are characterised along most of their length by subtle geomorphic signatures in areas of extensive forest cover and poor exposure. The NDpr was formed during the Early–Middle Quaternary, after the deposition of the Neogene basin fill. During the Middle– Late Quaternary, the NW–SE fault zones that bound northern Evia have been the main active structures, truncating and uplifting the NDpr to a perched position in relation to the northern Gulf of Evia graben and the submarine basin on the Aegean side of the island. -

ANNUAL FINANCIAL REPORT for the Period
TERNA ENERGY Industrial Commercial Technical Societe Anonyme 85 Mesogeion Ave., 115 26 Athens, Greece Societe Anonyme Reg. No. 318/06/Β/86/28 ANNUAL FINANCIAL REPORT for the period from 1st January to 31st December 2019 In accordance with Article 4, Law 3556/2007 and the relevant Executive Decisions of the Hellenic Capital Market Commission Board of Directors TERNA ENERGY GROUP Annual Financial Report for 2019 CONTENTS Ι. REPRESENTATIONS OF THE MEMBERS OF THE BOARD OF DIRECTORS ................................................................................ 4 ΙΙ. INDEPENDENT AUDITOR’S REPORT ...................................................................................................................................... 5 ΙΙΙ. ANNUAL MANAGEMENT REPORT OF THE BOARD OF DIRECTORS OF “TERNA ENERGY S.A.” ON CONSOLIDATED AND SEPARATE FINANCIAL STATEMENTS FOR FINANCIAL YEAR 2019 ........................................................................................... 10 CORPORATE GOVERNANCE STATEMENT ................................................................................................................................ 30 ΙV. ANNUAL SEPARATE AND CONSOLIDATED FINANCIAL STATEMENTS ............................................................................... 40 NOTES TO FINANCIAL STATEMENTS ...................................................................................................................................... 51 1 GENERAL INFORMATION ABOUT THE GROUP ..............................................................................................................