Heavy Metals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗
Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗ I examine the estimated economic, ecological, and food security effects of future fishery management reform in Asia. Without climate change, most Asian fisheries stand to gain substantially from reforms. Optimizing fishery management could increase catch by 24% and profit by 34% over business- as-usual management. These benefits arise from fishing some stocks more conservatively and others more aggressively. Although climate change is expected to reduce carrying capacity in 55% of Asian fisheries, I find that under climate change large benefits from fishery management reform are maintained, though these benefits are heterogeneous. The case for reform remains strong for both catch and profit, though these numbers are slightly lower than in the no-climate change case. These results suggest that, to maximize economic output and food security, Asian fisheries will benefit substantially from the transition to catch shares or other economically rational fishery management institutions, despite the looming effects of climate change. Keywords: Asia, climate change, fisheries, rights-based management JEL codes: Q22, Q28 I. Introduction Global fisheries have diverged sharply over recent decades. High governance, wealthy economies have largely adopted output controls or various forms of catch shares, which has helped fisheries in these economies overcome inefficiencies arising from overfishing (Worm et al. 2009) and capital stuffing (Homans and Wilen 1997), and allowed them to turn the corner toward sustainability (Costello, Gaines, and Lynham 2008) and profitability (Costello et al. 2016). But the world’s largest fishing region, Asia, has instead largely pursued open access and input controls, achieving less long-run fishery management success (World Bank 2017). -

Data Collection and Size Sampling on Neritic Tuna Fisheries in Andaman
IOTC–2018–WPNT08–15 Data Collection and size sampling on Neritic Tuna Fisheries in Andaman Sea Kanokwan Maeroh, Sichon Hoimuk, Suchart Inthong, and Supachai Rodpradit Upper Andaman Sea Fisheries Research and Development Center (Phuket) 77 Moo 7 Vichit Sub-District, Muang District, Phuket Province 83000 Tel. 0 7639 1138-40 e-mail: [email protected] Abstract In the Andaman Sea Coast of Thailand, there are many kinds of fishing gears can catch neritic tuna but most of it were caught by purse seine. The other gears are Otter board trawl, Anchovy falling nets and Squid Falling nets. There are 3 organizations along Andaman sea under Marine Fisheries Research and Development Division responsible to collect the data on fish composition and size distribution, especially for neritic tuna and others importantly economic fish, from more than 10 types of fishing gears. All kind of fishing gears in which both commercial and artisanal fisheries were conducted fisheries data for 3-5 days a month. There are 7 organizations along Andaman sea under Fishing and Fleets Management Division responsible to collected fishing data from logbook and catches landing of marine fish, to recorded and reported the data to Fishing-Info data base. Study on CPUE and MSY were conducted by Fisheries Statistics Analysis and Research Group and Fisheries Resources Assessment Group which are under the Department of Fishery. The purse seine is the mainly fishing gear for pelagic species. Pair trawls and otter board trawls are the main fishing gears for demersal species. The other gears which have a specific name to target species, such as gill nets, hand line and long line. -

Fishes of Terengganu East Coast of Malay Peninsula, Malaysia Ii Iii
i Fishes of Terengganu East coast of Malay Peninsula, Malaysia ii iii Edited by Mizuki Matsunuma, Hiroyuki Motomura, Keiichi Matsuura, Noor Azhar M. Shazili and Mohd Azmi Ambak Photographed by Masatoshi Meguro and Mizuki Matsunuma iv Copy Right © 2011 by the National Museum of Nature and Science, Universiti Malaysia Terengganu and Kagoshima University Museum All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means without prior written permission from the publisher. Copyrights of the specimen photographs are held by the Kagoshima Uni- versity Museum. For bibliographic purposes this book should be cited as follows: Matsunuma, M., H. Motomura, K. Matsuura, N. A. M. Shazili and M. A. Ambak (eds.). 2011 (Nov.). Fishes of Terengganu – east coast of Malay Peninsula, Malaysia. National Museum of Nature and Science, Universiti Malaysia Terengganu and Kagoshima University Museum, ix + 251 pages. ISBN 978-4-87803-036-9 Corresponding editor: Hiroyuki Motomura (e-mail: [email protected]) v Preface Tropical seas in Southeast Asian countries are well known for their rich fish diversity found in various environments such as beautiful coral reefs, mud flats, sandy beaches, mangroves, and estuaries around river mouths. The South China Sea is a major water body containing a large and diverse fish fauna. However, many areas of the South China Sea, particularly in Malaysia and Vietnam, have been poorly studied in terms of fish taxonomy and diversity. Local fish scientists and students have frequently faced difficulty when try- ing to identify fishes in their home countries. During the International Training Program of the Japan Society for Promotion of Science (ITP of JSPS), two graduate students of Kagoshima University, Mr. -

Surat Thani Blue Swimming Crab Fishery Improvement Project
Surat Thani Blue Swimming Crab Fishery Improvement Project -------------------------------------------------------------------------------------------------------------------------------------- Milestone 33b: Final report of bycatch research Progress report: The study of fishery biology, socio-economic and ecosystem related to the restoration of Blue Swimming Crab following Fishery improvement program (FIP) in Bandon Bay, Surat Thani province. Amornsak Sawusdee1 (1) The Center of Academic Service, Walailak University, Tha Sala, Nakhon Si Thammarat, 80160 The results of observation of catching BSC by using collapsible crab trap and floating seine. According to the observation of aquatic animal which has been caught by main BSC fishing gears; floating seine and collapsible crab trap, there were 176 kind of aquatic animals. The catch aquatic animals are shown in the table1. In this study, aquatic animal was classified into 11 Groups; Blue Swimming Crab (Portunus Pelagicus), Coelenterata (coral animals, true jellies, sea anemones, sea pens), Helcionelloida (clam, bivalve, gastropod), Cephalopoda (sqiud, octopus), Chelicerata (horseshoe crab), Hoplocari(stomatopods), Decapod (shrimp), Anomura (hermit crab), Brachyura (crab), Echinoderm (sea cucambers, sea stars, sea urchins), Vertebrata (fish). Vertebrata was the main group that was captured by BSC fishing gears, more than 70 species. Next are Helcionelloida and Helcionelloida 38 species and 29 species respectively. The sample that has been classified were photographed and attached in appendix 1. However, some species were classified as unknow which are under the classification process and reconcile. There were 89 species that were captured by floating seine. The 3 main group that were captured by this fishing gear are Vertebrata (34 species), Brachyura (20 species) Helcionelloida and Echinoderm (10 Species). On the other hand, there were 129 species that were captured by collapsible crab trap. -

General Knowledge of Pakistan Sanabil Educare Bhiri Shah Rahman
1 | P a g e General Knowledge of Pakistan by Sanabil Educare Bhiri Shah Rahman General Knowledge of Pakistan Sanabil Educare Bhiri Shah Rahman Q1: Who suggested name of Pakistan? Ans: Chaudhary Rahmat Ali. Q2: Who is known as father of Pakistan? Ans: Quaid-e-Azam- known as "The Great Leader" and Baba-e-Qaum (Father of the Nation). Q3: Who wrote the national anthem of Pakistan? Ans: Hafeez Jullundhri Q4: When was Pakistani national anthem first sung and where? Ans: Pakistani national anthem was broadcast publicly for the first time on Radio Pakistan on 13 August 1954, sung by Hafeez Jullundhri himself. Q5: Who made Pakistan flag first time? Ans: Amiruddin Kidwai. Q6: Who of Pakistan The first capital? Ans: The first capital of Pakistan was the coastal city of Karachi. Q7: Who was the first president of Pakistan? Ans: Iskander Mirza. Q8: Who was the first prime minister of Pakistan? Ans: Liaquat Ali Khan. Q9: Who was the first governor general of Pakistan? Ans: Muhammad Ali Jinnah. Q10: Who was the first chief justice of Pakistan? Ans: Zaheer Jamali. Q11: Who was the first female prime minister of Pakistan? Ans: Benazir Bhutto. Q12: Who was the first female speaker of the national assembly of Pakistan? Ans: Fehmida Mirza (PPPP) Q13: Who was the first chief of army staff of Pakistan? 2 | P a g e General Knowledge of Pakistan by Sanabil Educare Bhiri Shah Rahman Ans: Sir Frank Messervy- (15 August 1947 to 10 February 1948. Q14: Who was the first Chief of Air Staff of Pakistan? Ans: Allan Perry-Keene (15 August 1947 to 17 February 1949). -
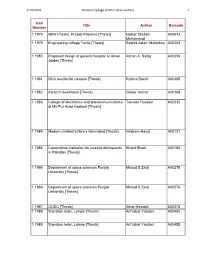
National Colege of Art Thesis List.Xlsx
4/16/2010 National College of Arts Library‐Lahore 1 Call Title Author Barcode Number 1 1975 MPA's hostel, Punjab Province [Thesis] Qaiser Ghulam A00573 Muhammad 1 1979 Engineering college Taxila [Thesis] Rashid Aslam Makhdum A00353 1 1980 Proposed design of general hospital at Aman Adnan A. Sadiq A00359 Jordan [Thesis] 1 1981 NCA residential campus [Thesis] Robina Bashir A00365 1 1982 Karachi Gymkhana [Thesis] Qaiser Ashrat A00368 1 1983 College of electronics and telecommunications Tanveer Hussain A00330 at Mir Pur Azad Kashmir [Thesis] 1 1984 Modern children's library Islamabad [Thesis] Nadeem Hayat A00121 1 1985 Correctional institution for juvenile delinquents Khalid Bhatti A00180 in Paksitan [Thesis] 1 1986 Department of space sciences Punjab Masud S Zaidi A00275 University [Thesis] 1 1986 Department of space sciences Punjab Masud S Zaidi A00276 University [Thesis] 1 1987 OGDC [Thesis] Athar Hussain A00375 1 1988 Sheraton hotel, Lahore [Thesis] Arif Iqbal Yazdani A00454 1 1988 Sheraton hotel, Lahore [Thesis] Arif Iqbal Yazdani A00455 4/16/2010 National College of Arts Library‐Lahore 2 1 1989 Engineering college Multan [Thesis] Razi-ud-Din A00398 1 1990 Islamabad hospital [Thesis] Nasir Iqbal A00480 1 1990 Islamabad hospital [Thesis] Nasir Iqbal A00492 1 1992 International Islamic University Islamabad Muhammad Javed A00584 [Thesis] 1 1994 Islamabad railway terminal: Golra junction Farah Farooq A00608 [Thesis] 1 1995 Community Facilities for Real People: Filling Ayla Musharraf A00619 Doxiadus Blanks [Thesis] 1 1995 Community Facilities -

Chapter 4 Environmental Management Consultants Ref: Y8LGOEIAPD ESIA of LNG Terminal, Jetty & Extraction Facility - Pakistan Gasport Limited
ESIA of LNG Terminal, Jetty & Extraction Facility - Pakistan Gasport Limited 4 ENVIRONMENTAL BASELINE OF THE AREA Baseline data being presented here pertain to the data collected from various studies along the physical, biological and socio-economic environment coast show the influence of NE and SW monsoon of the area where the proposed LNG Jetty and land winds. A general summary of meteorological and based terminal will be located, constructed and hydrological data is presented in following operated. Proposed location of project lies within the section to describe the coastal hydrodynamics of boundaries of Port Qasim Authority and very near the area under study. the Korangi Fish Harbour. Information available from electronic/printed literature relevant to A- Temperature & Humidity baseline of the area, surrounding creek system, Port Qasim as well as for Karachi was collected at the The air temperature of Karachi region is outset and reviewed subsequently. This was invariably moderate due to presence of sea. followed by surveys conducted by experts to Climate data generated by the meteorological investigate and describe the existing socio-economic station at Karachi Air Port represents climatic status, and physical scenario comprising conditions for the region. The temperature hydrological, geographical, geological, ecological records for five years (2001-2005) of Karachi city and other ambient environmental conditions of the are being presented to describe the weather area. In order to assess impacts on air quality, conditions. Table 4.1 shows the maximum ambient air quality monitoring was conducted temperatures recorded during the last 5 years in through expertise provided by SUPARCO. The Karachi. baseline being presented in this section is the extract of literature review, analyses of various samples, Summer is usually hot and humid with some surveys and monitoring. -

Eia- Final Report Sawera Residency
DUA Associates f Environmental Impact Assessment (EIA) SAWERA RESIDENCY I Plot No. 10 at Sheet No. FT-2 Frere Town Quarters, Karachi Final Report December 2020 0 Dua Associates Environmental Impact Assessment (EIA) SAWERA RESIDENCY I Plot No. 10 at Sheet No. FT-2 Frere Town Quarters, Karachi FINAL REPORT December 2020 Dua Associates Environmental Impact Environment, Health & Safety Services Assessment (EIA) 701, Greens Two Building Plot No. 155-S, Sir Syed Road, P.E.C.H.S Block-2, Karachi Contact # +92-300-0320332 WhatsApp # +92-345-2447202 Email: [email protected] SAWERA RESIDENCY I Web: www.ehss.info Plot No. 10 at Sheet No. FT-2 Frere Town Quarters, Karachi Prepared for: DUA Associates Office # 801, Balad Trade Center, Plot # 118, BMCHS, Block 03, Karachi Prepared by: EHS Services Date: December 2020 This document is intended only for the use of the individual or entity for which it was prepared and may contain information that is privileged, confidential and exempt from disclosure under applicable law. Any dissemination, distribution or copying of this document is strictly prohibited. SAWERA RESIDENCY - I Environmental Impact Plot No. 10 at Sheet No. FT-2 Frere Town Quarters, Karachi Assessment (EIA) 1. Introduction 17 1.1 Project Overview 18 1.2 Need for Environmental Impact Assessment (EIA) 21 1.3 Methodology for Environmental Assessment 22 2. Description of Project 24 2.1 Statutory Approvals 24 2.2 The Project 24 2.3 Construction & Commissioning 39 2.3.1 Pre-Construction Phase 39 2.3.2 Construction Phase 39 2.3.3 Standard Operating -

Juveniles of the Torpedo Scad, Megalaspis Cordyla
NOTE UPDATE AND CORRECTION Juveniles of the Torpedo Scad, Megalaspis cordyla (Teleostei: Carangidae), schooling with venomous catfishes (Plotosidae): a new case of mimicry and an identification correction WILLIAM F. SMITH-VANIZ Florida Museum of Natural History, University of Florida, Gainesville, FL 32611, USA E-mail: [email protected] Key words: fishes, ichthyology, Batesian mimicry, ecology, symbiosis, mutualism, coral reefs, Indonesia, Indian Ocean, Indo-Pacific. Citation: Smith-Vaniz, W.F. (2018) Juveniles of the Torpedo Scad, Megalaspis cordyla (Teleostei: Carangidae), schooling with venomous catfishes (Plotosidae): a new case of mimicry and an identification correction. Journal of the Ocean Science Foundation, 30, 105–107. doi: http://dx.doi.org/10.5281/zenodo.1467454 Smith-Vaniz et al. (2018) reported a case of mimicry between juveniles of Caranx bucculentus Alleyne & Macleay (Carangidae) and Plotosus lineatus (Thunberg) (Plotosidae) in this volume of the Journal of the Ocean Science Foundation (p. 82). The identifications were based solely on photographs of schooling juveniles at Lembeh Strait, Indonesia. Soon after the on-line version of the paper became available, new information made it obvious that the carangid had been misidentified. A color image of an approximately 5 cm fork length juvenile of C. bucculentus from Western Australia was kindly sent to me by John Pogonoski. This specimen has a much deeper body than the mimic carangid and its coloration is very different. Subsequently, I received 4 color photographs of what was clearly the same carangid illustrated in Smith-Vaniz et al. (2018) in association with Plotosus schools. Journal of the Ocean Science Foundation, 30, 105–107 (2018) 105 One of these photographs (Fig. -
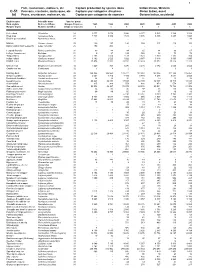
Fish, Crustaceans, Molluscs, Etc Capture
1 Fish, crustaceans, molluscs, etc Capture production by species items Indian Ocean, Western C-51 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Océan Indien, ouest (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Océano Indico, occidental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 1998 1999 2000 2001 2002 2003 2004 Nombre inglés Nombre científico Grupo de especies t t t t t t t Kelee shad Hilsa kelee 24 3 077 3 076 3 896 4 277 5 525 2 306 3 208 Hilsa shad Tenualosa ilisha 24 7 747 6 482 4 545 3 846 6 146 8 257 4 024 Bloch's gizzard shad Nematalosa nasus 24 ... ... ... ... ... ... 93 Milkfish Chanos chanos 25 139 137 125 154 117 113 101 Barramundi(=Giant seaperch) Lates calcarifer 25 196 204 - - - - - Leopard flounder Bothus pantherinus 31 90 84 84 65 84 96 67 Lefteye flounders nei Bothidae 31 9 27 129 125 88 73 78 Tonguefishes Cynoglossidae 31 2 158 2 066 2 137 1 923 1 989 1 383 984 Indian halibut Psettodes erumei 31 3 192 2 520 3 022 2 957 3 118 3 365 4 095 Flatfishes nei Pleuronectiformes 31 17 474 12 851 24 720 11 489 15 573 15 924 11 325 Unicorn cod Bregmaceros mcclellandi 32 1 449 743 1 470 2 435 2 372 2 643 2 624 Gadiformes nei Gadiformes 32 7 5 2 1 - 1 3 Bombay-duck Harpadon nehereus 33 144 865 146 663 133 221 141 082 100 366 101 936 116 452 Greater lizardfish Saurida tumbil 33 6 064 3 710 3 485 3 894 4 247 3 634 2 655 Brushtooth lizardfish Saurida undosquamis 33 34 20 30 32 20 11 18 Lizardfishes nei Synodontidae 33 20 221 20 871 17 894 12 433 12 447 10 715 13 833 Giant catfish Arius thalassinus 33 315 309 554 480 470 574 597 Sea catfishes nei Ariidae 33 99 932 98 467 78 375 89 308 89 941 78 788 81 972 Sabre squirrelfish Sargocentron spiniferum 33 34 53 46 97 96 135 114 Flathead grey mullet Mugil cephalus 33 69 89 60 34 37 40 46 Klunzinger's mullet Liza klunzingeri 33 .. -

3. MARINE FISHERY RESOURCES the Coastal Waters of Cambodia
3. MARINE FISHERY RESOURCES The coastal waters of Cambodia support a large number of marine fish and invertebrate species. Try (2003) gives the scientific, English and Kmer names for those marine species recorded from the country: 476 species of marine finfish, 20 species of marine crabs, 42 species of marine gastropods and 24 species of marine bivalves. In the marine fisheries statistics published by the Department of Fisheries (DoF, 2002), nine different groups are given. The 2001 landings of these groups by province/municipality are provided in Table 2 below. Little quantitative information is available on the composition of the finfish component. Table 2 Marine fishery landings recorded by DoF, 2001 Province Krill Krill Ray Sea Fish Crab Shrimp Total Snail Trash fish cucumber Cephalopod Blood cockle Slipper lobster Kampot 2 703 1 786 284 165 247 0 870 176 199 0 0 6 430 Sihanouk 6 943 4 287 1 730 0 1 496 40 897 1 236 226 210 0 17 065 Koh 7 104 4 764 1 606 42 604 0 1 410 1 082 762 0 26 17 400 Kong Kep 123 10 42 2 8 0 285 0 0 470 123 1 063 Total 16 873 10 847 3 662 209 2 355 40 3 462 2 494 1 187 680 149 41 958 Source: DoF, 2002. These recorded 2001 landings are depicted in Figure 2 below. It can be seen that about two- thirds of the catch consists of “fish” and “trash fish”. Figure 2. Marine fishery landings recorded by DoF, 2001 Fish Trash fish Shrimp Other Cephalopod Crab Snail Cockle Source: Table 2. -

48307-001: Engro Fast Track LNG Regasification Project
Draft Environmental Impact Assessment Volume 1 Project Number: 48307-001 July 2014 PAK: Engro Fast Track LNG Regasification Project Prepared by Environmental Management Consultants (EMC) for Engro Elengy Terminal Private Limited The environmental impact assessment is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. Your attention is directed to the “Terms of Use” section of this website. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. Elengy Terminal Pakistan Limited Environmental & Social Impact Assessment Proposed LNG Import Terminal Project, Port Qasim-Karachi July 2014 ENVIRONMENTAL MANAGEMENT CONSULTANTS 503, Anum Estate, Opp. Duty Free Shop, Main Shahrae Faisal, Karachi. Phones: 9221-4311466, 4311467, Fax: 9221-4311467. E-mail: [email protected], [email protected] Website: www.emc.com.pk Elengy Terminal Proposed LNG Import Terminal Project, Port Qasim-Karachi Pakistan Limited ESIA Report Executive Summary INTRoDUCTIoN AND oBJECTIVES This Environmental & Social Impact Assessment (ESIA) evaluates the potential environmental, social, economic, cultural, and natural impacts of the proposed Liquefied Natural Gas (LNG) Import Floating Terminal Project. Environmental Management Consultants (EMC) Pakistan has been contracted as a third party consultant by Elengy Terminal Pakistan Limited (hereinafter referred as proponent) to conduct a detailed assessment (ESIA) of the proposed LNG project.