Aneura Maxima – a Liverwort New to Poland
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aquatic and Wet Marchantiophyta, Order Metzgeriales: Aneuraceae
Glime, J. M. 2021. Aquatic and Wet Marchantiophyta, Order Metzgeriales: Aneuraceae. Chapt. 1-11. In: Glime, J. M. Bryophyte 1-11-1 Ecology. Volume 4. Habitat and Role. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 11 April 2021 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 1-11: AQUATIC AND WET MARCHANTIOPHYTA, ORDER METZGERIALES: ANEURACEAE TABLE OF CONTENTS SUBCLASS METZGERIIDAE ........................................................................................................................................... 1-11-2 Order Metzgeriales............................................................................................................................................................... 1-11-2 Aneuraceae ................................................................................................................................................................... 1-11-2 Aneura .......................................................................................................................................................................... 1-11-2 Aneura maxima ............................................................................................................................................................ 1-11-2 Aneura mirabilis .......................................................................................................................................................... 1-11-7 Aneura pinguis .......................................................................................................................................................... -

A Taxonomic Revision of Aneuraceae (Marchantiophyta) from Eastern Africa with an Interactive Identification Key
cryptogamie Bryologie 2019 ● 41 ● 2 DIRECTEUR DE LA PUBLICATION : Bruno David, Président du Muséum national d’Histoire naturelle RÉDACTEURS EN CHEF / EDITORS-IN-CHIEF : Denis LAMY, Michelle Price ASSISTANTS DE RÉDACTION / ASSISTANT EDITORS : Marianne SALAÜN ([email protected]) MISE EN PAGE / PAGE LAYOUT : Marianne SALAÜN RÉDACTEURS ASSOCIÉS / ASSOCIATE EDITORS Biologie moléculaire et phylogénie / Molecular biology and phylogeny Bernard GOFFINET Department of Ecology and Evolutionary Biology, University of Connecticut (United States) Mousses d’Europe / European mosses Isabel DRAPER Centro de Investigación en Biodiversidad y Cambio Global (CIBC-UAM), Universidad Autónoma de Madrid (Spain) Francisco LARA GARCÍA Centro de Investigación en Biodiversidad y Cambio Global (CIBC-UAM), Universidad Autónoma de Madrid (Spain) Mousses d’Afrique et d’Antarctique / African and Antarctic mosses Rysiek OCHYRA Laboratory of Bryology, Institute of Botany, Polish Academy of Sciences, Krakow (Pologne) Bryophytes d’Asie / Asian bryophytes Rui-Liang ZHU School of Life Science, East China Normal University, Shanghai (China) Bioindication / Biomonitoring Franck-Olivier DENAYER Faculté des Sciences Pharmaceutiques et Biologiques de Lille, Laboratoire de Botanique et de Cryptogamie, Lille (France) Écologie des bryophytes / Ecology of bryophyte Nagore GARCÍA MEDINA Department of Biology (Botany), and Centro de Investigación en Biodiversidad y Cambio Global (CIBC-UAM), Universidad Autónoma de Madrid (Spain) COUVERTURE / COVER : From top left, to bottom right, by -

Wikstrom2009chap13.Pdf
Liverworts (Marchantiophyta) Niklas Wikströma,*, Xiaolan He-Nygrénb, and our understanding of phylogenetic relationships among A. Jonathan Shawc major lineages and the origin and divergence times of aDepartment of Systematic Botany, Evolutionary Biology Centre, those lineages. Norbyvägen 18D, Uppsala University, Norbyvägen 18D 75236, Altogether, liverworts (Phylum Marchantiophyta) b Uppsala, Sweden; Botanical Museum, Finnish Museum of Natural comprise an estimated 5000–8000 living species (8, 9). History, University of Helsinki, P.O. Box 7, 00014 Helsinki, Finland; Early and alternative classiA cations for these taxa have cDepartment of Biology, Duke University, Durham, NC 27708, USA *To whom correspondence should be addressed (niklas.wikstrom@ been numerous [reviewed by Schuster ( 10)], but the ebc.uu.se) arrangement of terminal taxa (species, genera) into lar- ger groups (e.g., families and orders) based on morpho- logical criteria alone began in the 1960s and 1970s with Abstract the work of Schuster (8, 10, 11) and Schljakov (12, 13), and culminated by the turn of the millenium with the work Liverworts (Phylum Marchantiophyta) include 5000–8000 of Crandall-Stotler and Stotler (14). 7 ree morphological species. Phylogenetic analyses divide liverworts into types of plant bodies (gametophytes) have generally been Haplomitriopsida, Marchantiopsida, and Jungerman- recognized and used in liverwort classiA cations: “com- niopsida. Complex thalloids are grouped with Blasiales in plex thalloids” including ~6% of extant species diversity Marchantiopsida, and leafy liverworts are grouped with and with a thalloid gametophyte that is organized into Metzgeriidae and Pelliidae in Jungermanniopsida. The distinct layers; “leafy liverworts”, by far the most speci- timetree shows an early Devonian (408 million years ago, ose group, including ~86% of extant species diversity and Ma) origin for extant liverworts. -
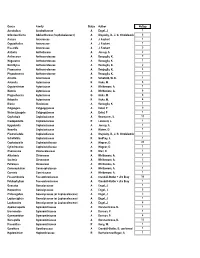
Family Status Author Nospp Acrobolbus Acrobolbaceae a Engel, J
Genus Family Status Author NoSpp Acrobolbus Acrobolbaceae A Engel, J. 1 Odontoschisma Adelanthaceae (Cephaloziaceae?) A Krayesky, D., J. G. Chmielewski 6 Aneura Aneuraceae A J. Faubert 1 Cryptothallus Aneuraceae A J. Faubert 1 Riccardia Aneuraceae A J. Faubert 5 Anthelia Antheliaceae A Jessup, S. 2 Anthoceros Anthocerotaceae A Renzaglia, K. 7 Megaceros Anthocerotaceae A Renzaglia, K. 1 Notothylas Anthocerotaceae A Renzaglia, K. 2 Phaeoceros Anthocerotaceae A Renzaglia, K. 4 Phymatoceros Anthocerotaceae A Renzaglia, K. 1 Arnellia Arnelliaceae R Schofield, W. B. 1 Asterella Aytoniaceae R Hicks, M. 8 Cryptomitrium Aytoniaceae A Whittemore, A. 1 Mannia Aytoniaceae A Whittemore, A. 6 Plagiochasma Aytoniaceae G Hicks, M. 6 Reboulia Aytoniaceae R Hicks, M. 4 Blasia Blasiaceae A Renzaglia, K. 1 Calypogeia Calypogejaceae A Eckel, P 9 Metacalypogeia Calypogejaceae A Eckel, P 1 Cephalozia Cephaloziaceae A Newmaster, S. 11 Cladopodiella Cephaloziaceae R Leonardi, L. 1 Hygrobiella Cephaloziaceae A Jessup, S. 1 Nowellia Cephaloziaceae A Waters, D. 1 Pleurocladula Cephaloziaceae A Krayesky, D., J. G. Chmielewski 1 Schofieldia Cephaloziaceae R Godfrey, J. 1 Cephaloziella Cephaloziellaceae A Wagner, D. 21 Cylindrocolea Cephaloziellaceae A Wagner, D. 1 Chonecolea Chonecoleaceae R Breil, D. 1 Athalamia Cleveaceae A Whittemore, A. 1 Sauteria Cleveaceae A Whittemore, A. 2 Peltolepis Cleveaceae A Whittemore, A. 1 Conocephalum Conocephalaceae A Whittemore, A. 1 Corsinia Corsiniaceae A Whittemore, A. 1 Fossombronia Fossombroniaceae A Crandall-Stotler + Jim Bray 10 Petalophyllum Fossombroniaceae A Crandall-Stotler + Jim Bray 1 Geocalyx Geocalycaceae A Engel, J. 1 Harpanthus Geocalycaceae A Engel, J. 3 Chiloscyphus Geocalycaceae (or Lophocoleaceae) A Engel, J. 3 Leptoscyphus Geocalycaceae (or Lophocoleaceae) A Engel, J. -

Article ISSN 2381-9685 (Online Edition)
Bry. Div. Evo. 043 (1): 284–306 ISSN 2381-9677 (print edition) DIVERSITY & https://www.mapress.com/j/bde BRYOPHYTEEVOLUTION Copyright © 2021 Magnolia Press Article ISSN 2381-9685 (online edition) https://doi.org/10.11646/bde.43.1.20 Advances in understanding of mycorrhizal-like associations in bryophytes SILVIA PRESSEL1*, MARTIN I. BIDARTONDO2, KATIE J. FIELD3 & JEFFREY G. DUCKETT1 1Life Sciences Department, The Natural History Museum, Cromwell Road, London SW7 5BD, UK; �[email protected]; https://orcid.org/0000-0001-9652-6338 �[email protected]; https://orcid.org/0000-0001-7101-6673 2Imperial College London and Royal Botanic Gardens, Kew TW9 3DS, UK; �[email protected]; https://orcid.org/0000-0003-3172-3036 3 Department of Animal and Plant Sciences, University of Sheffield, Sheffield, S10 2TN, UK; �[email protected]; https://orcid.org/0000-0002-5196-2360 * Corresponding author Abstract Mutually beneficial associations between plants and soil fungi, mycorrhizas, are one of the most important terrestrial symbioses. These partnerships are thought to have propelled plant terrestrialisation some 500 million years ago and today they play major roles in ecosystem functioning. It has long been known that bryophytes harbour, in their living tissues, fungal symbionts, recently identified as belonging to the three mycorrhizal fungal lineages Glomeromycotina, Ascomycota and Basidiomycota. Latest advances in understanding of fungal associations in bryophytes have been largely driven by the discovery, nearly a decade ago, that early divergent liverwort clades, including the most basal Haplomitriopsida, and some hornworts, engage with a wider repertoire of fungal symbionts than previously thought, including endogonaceous members of the ancient sub-phylum Mucoromycotina. -

Differentiation and Genetic Variability of Three Cryptic Species Within the Aneura Pinguis Complex (Jungermanniidae, Marchantiophyta)
Cryptogamie, Bryologie, 2016, 37 (1): 3-18 © 2016 Adac. Tous droits réservés Differentiation and genetic variability of three cryptic species within the Aneura pinguis complex (Jungermanniidae, Marchantiophyta) Alina Bączkiewicz* & Katarzyna Buczkowska Department of Genetics, a. Mickiewicz university, umultowska 89, 61-614 Poznań, Poland ABSTRACT – 1652 individuals of aneura pinguis from Poland were surveyed for variation in 12 putative gene loci. Based on isozyme data, we distinguished three cryptic species. No evidence for gene flow between these species was found. To date, no qualitative morphological characters are available, which would allow delimitation of the cryptic species of a. pinguis. Hence, these species are not formally described, but assigned as cryptic species A, B, and C. The mean genetic distance (D) between them is 1.3393. The highest genetic variation within populations (Hs) was found in species A, and the lowest in species B. Individual species of a. pinguis differ in their habitat preferences. Species A is the most common, it occurs mostly in the Western Carpathians, grows mainly on calcareous rocks and humus. Species B is the most frequent in the Eastern Carpathians on clay soil. Species C is the rarest, it can be found both in lowlands and mountains, but mainly in lowlands and on various substrata. All studied cryptic species occur partly sympatrically. Liverworts / Aneura pinguis / cryptic species / allozyme / cpDNA-trnL / genetic variation / geographic distribution / ecological preferences. RéSuMé – Différentiation and variabilité génétique de trios espèces cryptiques dans le complexe Aneura pinguis (Jungermanniidae, Marchantiophyta). Des investigations portant sur 12 loci enzymatiques ont été menées sur une population de 1652 individus d’aneura pinguis collectés en Pologne. -

Arctic Biodiversity Assessment
310 Arctic Biodiversity Assessment Purple saxifrage Saxifraga oppositifolia is a very common plant in poorly vegetated areas all over the high Arctic. It even grows on Kaffeklubben Island in N Greenland, at 83°40’ N, the most northerly plant locality in the world. It is one of the first plants to flower in spring and serves as the territorial flower of Nunavut in Canada. Zackenberg 2003. Photo: Erik Thomsen. 311 Chapter 9 Plants Lead Authors Fred J.A. Daniëls, Lynn J. Gillespie and Michel Poulin Contributing Authors Olga M. Afonina, Inger Greve Alsos, Mora Aronsson, Helga Bültmann, Stefanie Ickert-Bond, Nadya A. Konstantinova, Connie Lovejoy, Henry Väre and Kristine Bakke Westergaard Contents Summary ..............................................................312 9.4. Algae ..............................................................339 9.1. Introduction ......................................................313 9.4.1. Major algal groups ..........................................341 9.4.2. Arctic algal taxonomic diversity and regionality ..............342 9.2. Vascular plants ....................................................314 9.4.2.1. Russia ...............................................343 9.2.1. Taxonomic categories and species groups ....................314 9.4.2.2. Svalbard ............................................344 9.2.2. The Arctic territory and its subdivision .......................315 9.4.2.3. Greenland ...........................................344 9.2.3. The flora of the Arctic ........................................316 -

Aneuraceae, Marchantiophytina)
European Journal of Taxonomy 273: 1–26 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2017.273 www.europeanjournaloftaxonomy.eu 2017 · Rabeau L. et al. This work is licensed under a Creative Commons Attribution 3.0 License. DNA Library of Life, research article New insights into the phylogeny and relationships within the worldwide genus Riccardia (Aneuraceae, Marchantiophytina) Lucile RABEAU 1,*, S. Robbert GRADSTEIN 2, Jean-Yves DUBUISSON 3, Martin NEBEL 4, Dietmar QUANDT 5 & Catherine REEB 6 1,2,3,6 Université Pierre et Marie Curie – Sorbonne Universités – Institut de Systématique, Évolution, Biodiversité, ISYEB, UMR 7205, CNRS-MNHN-UPMC-EPHE, Muséum national d’Histoire naturelle, 57 rue Cuvier, CP 39, 75005 Paris, France. 4 Staatliches Museum für Naturkunde Stuttgart, Rosenstein 1, 70191 Stuttgart, Germany. 5 Nees-Institut für Biodiversität der Pfl anzen, Rheinische Friedrich-Wilhelms-Universität Bonn, Meckenheimer Allee 170, 53115 Bonn, Germany. * Corresponding author: [email protected] 2 Email: [email protected] 3 Email: [email protected] 4 Email: [email protected] 5 Email: [email protected] 6 Email: [email protected] Abstract. With 280 accepted species, the genus Riccardia S.F.Gray (Aneuraceae) is one of the most speciose genera of simple thalloid liverworts. The current classifi cation of this genus is based on morphological and limited-sampling molecular studies. Very few molecular data are available and a comprehensive view of evolutionary relationships within the genus is still lacking. A phylogeny focusing on relationships within the large genus Riccardia has not been conducted. Here we propose the fi rst worldwide molecular phylogeny of the genus Riccardia, based on Bayesian inference and parsimony ratchet analyses of sequences from three plastid regions (psbA-trnH, rps4, trnL-F). -

Application of Different DNA Markers in Studies on Cryptic Species of Aneura Pinguis (Jungermanniopsida, Metzgeriales)
Cryptogamie, Bryologie, 2008, 29 (1): 3-21 © 2008 Adac. Tous droits réservés Application of different DNA markers in studies on cryptic species of Aneura pinguis (Jungermanniopsida, Metzgeriales) AlinaB¥ CZKIEWICZa*, JakubSAWICKIb , KatarzynaBUCZKOWSKAa , KorneliaPOLOKc & RomanZIELIN SKIc a Department of Genetics, A. Mickiewicz University, Umultowska 89, 61-614 Pozna ¬ , Poland b Department of Botany and Nature Protection, University of Warmia and Mazury in Olsztyn, Plac fi ódzki 1, 10-727 Olsztyn, Poland c Department of Genetics, University of Warmia and Mazury in Olsztyn, Plac fi ódzki 3, 10-967 Olsztyn, Poland (Received 18 October 2006, accepted 12 April 2007) Abstract – The liverwort Aneura pinguis is represented in Europe by 4 cryptic species (A, B, C and D). They differ in morphology and ecological preferences. Three molecular marker systems (RAPD, ISJ and katG) were used to identify particular cryptic species. Ten samples of 4 cryptic species of A. pinguis and 2 samples of A. maxima (as an outgroup) were analysed. The 18 primers representing 3 different DNA marker categories revealed 147 bands, and 56 of them were species-specific. Among the cryptic species of A. pinguis , the greatest number of 21 specific bands was observed in species D, compared to 8 in species C, 7 in species B, 5 in species A, and 15 in A. maxima . The value of genetic similarity between analysed populations at the intraspecific level was I =0.90. In contrast, the I value between particular studied cryptic species ranged from 0.385 to 0.690. The similarities showed that species C and D are the most distinct, whereas species A and B are the most similar. -

(Aneuraceae, Hepaticopsida) in the Iberian Peninsula
J Hallori Bot. Lab. No. 97: 309- 3J6 (Jan. 2(05) MODELLING THE DISTRIBUTION OF CRYPTOTHALLUS MlRABILIS MALMB. (ANEURACEAE, HEPATICOPSIDA) IN THE IBERIAN PENINSULA CECIUA SERGIO', DAVID DRAPER2 AND CE:SAR GARCIA' ABSTRACT . Cryptothallus mirabilis Malmb. exhibits a specialised ecology and is a non-photosyn thetic Iiverwort. This species was first described for Scandinavia and till now was considered a north oceanic species. The discoveries of C. mirabilis in different localities in Portugal are the first reliable records of the species in southern Europe and represent an extension of its geographical range. [n fact, in Portugal C. mirabilis does not seem to be rare in areas with oceanic influence, mainly in wet forestry habitats. It is possibly widespread in the Iberian Peninsula because it is easily overlooked owing to its subterranean growth. The objective of this work is to give new localities which con tribute to an evaluation of its ecological requirements, to the definition of its biogeographical distrib ution in Portugal, and to present a predictive map for the Iberian Peninsula. The aim of the model is to identify the distribution pattern and also some potential places of occurrence in Spain and provide information about the environmental range of the species in order to improve actions for conserva tion. K EY WORDS: Cryptothallus mirabilis, ecology, predictive map, conservation, Iberian Peninsula INTRODUCTION The discoveries of Cryptothallus mirabilis Malmb. in Portugal (Sergio & Seneca 1997, Sergio & Garcia 1999) are the first reliable records of the species in southern Europe and represent an important extension of its geographic range. At that time, owing to our limited experience of the ecology of this species, the dis covery of C. -

Metzgeriaceae), a New Species from Late Cretaceous Japanese Amber
Hattoria 11: 13–21. 2020 Discovery of a simple thalloid liverwort Metzgeriites kujiensis (Metzgeriaceae), a new species from Late Cretaceous Japanese amber Tomoyuki KATAGIRI1 & Hisao SHINDEN2 1 Hattori Botanical Laboratory, Obi 6–1–26, Nichinan, Miyazaki 889–2535, Japan 2 Kuji Kohaku Co., Ltd., Kokuji-cho 19–156–133, Kuji, Iwate 028–0071, Japan Author for correspondence: Tomoyuki KATAGIRI, [email protected] Abstract An extinct liverwort species Metzgeriites kujiensis T.Katag., sp. nov. is described from the Late Cretaceous (Santonian, ca. 85 Ma) amber from the Kuji district, northern Honshu, Japan. It is characterized by thalloid plants with a distinct midrib and lamina, narrow thalli of 0.6–0.8 mm wide, regularly 1-pinnate ramification pattern, and presence of discoid branches. The new species represents the first record of the liverwort family Metzgeriaceae (Metzgeriales, Marchantiophyta) from the Late Cretaceous from East Asia. Introduction Kuji amber is the largest amber deposit in Japan and one of the most important sources for the reconstruction of terrestrial ecosystems in the Late Cretaceous East Asia continental margin, especially for revealing fauna and flora for microscopic species such as insects and bryophytes. In spite of its importance in paleontology and evolutionary biology and of their potential abundance with over 800 insect inclusions, few studies have been conducted on amber from the deposit. For its insect inclusions Kawakami et al. (1994) studied 23 specimens of fossil insects and Fursov et al. (2002) reported a fossil species of Mymarommatidae, an extremely rare family of microscopic hymenopteran insects. Recently, Nakamine & Yamamoto (2018) discovered a remarkable new genus and species of thorny lacewing (Neuroptera, Rhachiberothidae) and stressed the importance of the insect inclusions in Kuji amber. -

Two New Species of Riccardia (Aneuraceae, Marchantiophyta) from Eastern Himalaya, India with Notes on the Genus in Sikkim
Taiwania 62(1): 33‒42, 2017 DOI: 10.6165/tai.2017.62.33 Two new species of Riccardia (Aneuraceae, Marchantiophyta) from Eastern Himalaya, India with notes on the genus in Sikkim Devendra SINGH1 and Devendra Kumar SINGH1, 2,* 1. Botanical Survey of India, Central National Herbarium, Howrah-711103, India , Howrah, West Bengal 711103 Kolkata, India. 2. Present Address: 305D, Saraswati Apartment, Gomti Nagar Extension, Lucknow - 226010, India. * Corresponding author Phone: +917080480720; Email: [email protected] (Manuscript received 18 July 2016; accepted 24 October 2016; online published 25 January 2017) ABSTRACT: The genus Riccardia Gray is represented in Sikkim by 10 species, including R. lachungensis D.Singh & D.K.Singh and R. udarii D.Singh & D.K.Singh described here as new from North and South district respectively. R. lachungensis is characterized by 9–10 mm long, 0.25–0.35 mm wide plants with usually bipinnate or sometimes tripinnately branched thalli with mamillate–papillate dorsal and ventral epidermal cells, mucilage papillae scattered on ventral surface of thallus, main thalli 10–12 cells thick in the middle with unistratose alar portion 1-celled, and the absence of gemmae. Whereas, R. udarii is distinct in dichotomously branched thalli with distinct midrib, or sometimes indistinct in apical portion, main thalli 6–8 (–9) cells thick in the middle in transverse section, unistratose alar portion of wing 4–9 cells wide and often presence of pigmented cells in the thallus. Sporophytic details in R. elata (Steph.) Schiffn. are provided for the first time and R. levieri Schiffn. is newly recorded from the State of Sikkim.