Micro-CT Screening of Old Shell Collections Helps To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Balea Perversa (L.), Un Escargot Peu Courant Et En Raréfaction Photo Pierre-Olivier COCHARD
Balea perversa (L.), un escargot peu courant et en raréfaction Photo Pierre-Olivier COCHARD Description de l’espèce Balea perversa (LINNAEUS, 1758) est un escargot terrestre de taille modeste, 8 à 10 mm de long pour 2,2 mm de large (KERNEY & CAMERON, 1999). Il fait partie de la famille des Clausiliidae. Les Clausiliidae se reconnaissent à leurs coquilles beaucoup plus longues que larges, généralement fusiformes, et leur enroulement sénestre (enroulement vers la gauche), phénomène très peu courant chez les mollusques. Cette famille est très diversifiée dans l’Est de l’Europe, les zones de montagnes en France, et n’est plus représentée dans l’Ouest de la France que par quelques espèces. En Normandie, environ 5 espèces sont actuellement connues, une ou deux autres signalées Photo Pierre-Olivier COCHARD Contrefort de Torigni 48 L’Argiope n°50 anciennement. La détermination du genre Clausilia, le genre le plus distribué dans la région, est un véritable casse-tête. Si tous les autres Clausiliidae de la Manche ont une coquille fusiforme, qui a son tour le plus large un peu au-dessous du milieu, B. perversa est une exception. Sa coquille est étroitement conique (sa partie la plus large se situe à la base). Autres exceptions, l’ouverture (la « bouche ») est sans dents (les Clausilia et Macrogastra en ont plusieurs), l’intérieur de la coquille ne présente pas de clausilium, sorte de lame calcaire en forme de cuillière. Enfin précision importante : l’épithète spécifique ne sous-entend rien d’autre de pervers chez cette espèce, que sa particularité inhabituelle d’être enroulée vers la gauche. -

(Mollusca, Gastropoda) of the Bulgarian Part of the Alibotush Mts
Malacologica Bohemoslovaca (2008), 7: 17–20 ISSN 1336-6939 Terrestrial gastropods (Mollusca, Gastropoda) of the Bulgarian part of the Alibotush Mts. IVAILO KANEV DEDOV Central Laboratory of General Ecology, 2 Gagarin Str., BG-1113 Sofia, Bulgaria, e-mail: [email protected] DEDOV I.K., 2008: Terrestrial gastropods (Mollusca, Gastropoda) of the Bulgarian part of the Alibotush Mts. – Malacologica Bohemoslovaca, 7: 17–20. Online serial at <http://mollusca.sav.sk> 20-Feb-2008. This work presents results of two years collecting efforts within the project “The role of the alpine karst area in Bulgaria as reservoir of species diversity”. It summarizes distribution data of 44 terrestrial gastropods from the Bulgarian part of Alibotush Mts. Twenty-seven species are newly recorded from the Alibotush Mts., 13 were con- firmed, while 4 species, previously known from the literature, were not found. In the gastropod fauna of Alibotush Mts. predominate species from Mediterranean zoogeographic complex. A large part of them is endemic species, and this demonstrates the high conservation value of large limestone areas in respect of terrestrial gastropods. Key words: terrestrial gastropods, distribution, Alibotush Mts., Bulgaria Introduction Locality 6: vill. Katuntsi, Izvorite hut, near hut, open The Alibotush Mts. (other popular names: Kitka, Gotseva ruderal terrain, under bark, 731 m a.s.l., coll. I. Dedov. Planina, Slavjanka) is one of the most interesting large Locality 7: vill. Katuntsi, tufa-gorge near village, 700 m limestone area in Bulgaria (Fig. 1). It occupies the part a.s.l., coll. I. Dedov, N. Simov. of the border region between Bulgaria and Greece with Locality 8: below Livade area, road between Goleshevo maximum elevation 2212 m (Gotsev peak). -

Snail Herbivory Decreases Cyanobacterial Abundance And
Snail herbivory decreases cyanobacterial abundance and lichen diversity along cracks of limestone pavements 1 2, 2 2 LARS FRO€BERG, PETER STOLL, ANETTE BAUR, AND BRUNO BAUR 1Botanical Museum, Department of Biology, O¨ stra Vallgatan 18, SE-223 61 Lund, Sweden 2Section of Conservation Biology, Department of Environmental Sciences, University of Basel, St. Johanns-Vorstadt 10, CH-4056 Basel, Switzerland Abstract. Herbivores are known to decrease plant species diversity in ecosystems with low productivity. Limestone pavements are low-productive habitats harboring specialized communities of cyanobacteria, and endo- and epilithic lichens exposed to extreme temperature and humidity fluctuations. Pavements of the Great Alvar (O¨ land, Sweden) are covered by free-living cyanobacteria giving the rock surface a dark color. Based on cyanobacterial abundance along the edges, two types of cracks intersecting the pavements have been described: Type one with abundant cyanobacteria and type two without cyanobacteria resulting in light-colored edges. Erosion and different lengths of inundation by melt water have been suggested to cause the conspicuous differences in community composition and hence color between cracks. We hypothesized that this pattern results from the grazing activity of the cyanobacteria- and lichen-feeding snail Chondrina clienta, which reduces cyanobacterial cover along light-colored cracks and facilitates endolithic lichens. Three dark and three light-colored cracks were investigated at each of three localities. Crack characteristics (i.e., aspect, width, depth and erosion) and snail density were assessed at the crack level. Cyanobacterial cover and lichen diversity were recorded in 1-cm sections, sampled every 5 cm along eight 160-cm-long transects per crack. -

On a Collection of Peruvian Neniinae (Mollusca: Gastropoda: Clausiliidae), with a Check-List and a Provisional Key to All the Peruvian Species Known
ON A COLLECTION OF PERUVIAN NENIINAE (MOLLUSCA: GASTROPODA: CLAUSILIIDAE), WITH A CHECK-LIST AND A PROVISIONAL KEY TO ALL THE PERUVIAN SPECIES KNOWN by F. E. LOOSJES and A. C. W. LOOSJES-VAN BEMMEL Loosjes, F. E., & A. C. W. Loosjes-van Bemmel: On a collection of Peruvian Nenuiinae (Mollus- ca, Gastropoda, Clausiliidae), with a check-list and a provisional key to all the Peruvian species known. Zool. Verh. Leiden 212, 5-ix-1984: 1-38, figs. 1-15, —ISSN 0024-1652. Key words: Mollusca; Clausiliidae; key; checklist; taxonomy; Peru. An annotated list is given of all Neniinae collected in 1975 by Dr. A. S. H. Breure in Peru. The localities that have been visited are also listed, together with the Neniinae collected there. Pseudo- gracilinenia gen. nov. is described for P. huallagana (Pilsbry, 1949) (type-species) and P.jolyi (O. Boettger, 1880); the latter species is only tentatively classified with Pseudogracilinenia because its anatomy is still unknown. Temesa (T.) breurei spec. nov. after eight specimens (shells) from 34 km N. of Junin. In addition a provisional key to all Peruvian Neniinae known is given, as well as a revised checklist. F. E. Loosjes & A. C. W. Loosjes-van Bemmel, Vossenlaan 4, 6705 CE Wageningen, The Ne- therlands. CONTENTS I. Introduction 3 II. The collection 4 II-1. List of localities, with species/subspecies collected 4 II-2. Species and subspecies 6 III. Provisional key to the Peruvian Neniinae, based on shell characters 17 IV. Revised list of Peruvian Neniinae 35 V. References 38 I. INTRODUCTION In 1975 Dr. A. -

The Slugs of Bulgaria (Arionidae, Milacidae, Agriolimacidae
POLSKA AKADEMIA NAUK INSTYTUT ZOOLOGII ANNALES ZOOLOGICI Tom 37 Warszawa, 20 X 1983 Nr 3 A n d rzej W ik t o r The slugs of Bulgaria (A rionidae , M ilacidae, Limacidae, Agriolimacidae — G astropoda , Stylommatophora) [With 118 text-figures and 31 maps] Abstract. All previously known Bulgarian slugs from the Arionidae, Milacidae, Limacidae and Agriolimacidae families have been discussed in this paper. It is based on many years of individual field research, examination of all accessible private and museum collections as well as on critical analysis of the published data. The taxa from families to species are sup plied with synonymy, descriptions of external morphology, anatomy, bionomics, distribution and all records from Bulgaria. It also includes the original key to all species. The illustrative material comprises 118 drawings, including 116 made by the author, and maps of localities on UTM grid. The occurrence of 37 slug species was ascertained, including 1 species (Tandonia pirinia- na) which is quite new for scientists. The occurrence of other 4 species known from publications could not bo established. Basing on the variety of slug fauna two zoogeographical limits were indicated. One separating the Stara Pianina Mountains from south-western massifs (Pirin, Rila, Rodopi, Vitosha. Mountains), the other running across the range of Stara Pianina in the^area of Shipka pass. INTRODUCTION Like other Balkan countries, Bulgaria is an area of Palearctic especially interesting in respect to malacofauna. So far little investigation has been carried out on molluscs of that country and very few papers on slugs (mostly contributions) were published. The papers by B a b o r (1898) and J u r in ić (1906) are the oldest ones. -

Predatory Poiretia (Stylommatophora, Oleacinidae) Snails: Histology and Observations
Vita Malacologica 13: 35-48 20 December 2015 Predatory Poiretia (Stylommatophora, Oleacinidae) snails: histology and observations Renate A. HELWERDA Naturalis Biodiversity Center, Darwinweg 2, 2333 CR Leiden, The Netherlands email: [email protected] Key words: Predation, predatory snails, drilling holes, radula, pedal gland, sole gland, acidic mucus ABSTRACT The Mediterranean species occur in rather dry, often rocky habitats, which are openly to sparsely vegetated. The predatory behaviour of Poiretia snails is studied. One However, they also occur in anthropogenically affected areas aspect of this behaviour is the ability to make holes in the such as gardens and parks (Kittel, 1997). The snails are main - shells of prey snails. The radula and the histology of the ly active at night and are hidden away under rocks and leaf mucous glands support the assumption that Poiretia secretes litter during the day, although they can also be found crawling acidic mucus to produce these holes. Observation of a around during daytime if the weather is rainy or cloudy and Poiretia compressa (Mousson, 1859) specimen yielded the moist (Wagner, 1952; Maassen, 1977; Kittel, 1997). During insight that its activities relied on the availability of moisture the hot summer months, Poiretia snails aestivate by burying and not on light conditions. It preyed on a wide range of snail themselves in soil or under rocks and sealing their apertures species, but only produced holes in shells when the aperture with an epiphragm (Kittel, 1997). was blocked. It usually stabbed its prey with a quick motion Poiretia snails prey on a wide variety of pulmonate snails. -

Molluscs, by Michael J
Cambourne New Settlement Iron Age and Romano-British settlement on the clay uplands of west Cambridgeshire Volume 2: Specialist Appendices Web Report 15 Molluscs, by Michael J. Allen Cambourne New Settlement Iron Age and Romano-British Settlement on the Clay Uplands of West Cambridgeshire By James Wright, Matt Leivers, Rachael Seager Smith and Chris J. Stevens with contributions from Michael J. Allen, Phil Andrews, Catherine Barnett, Kayt Brown, Rowena Gale, Sheila Hamilton-Dyer, Kevin Hayward, Grace Perpetua Jones, Jacqueline I. McKinley, Robert Scaife, Nicholas A. Wells and Sarah F. Wyles Illustrations by S.E. James Volume 2: Specialist Appendices Part 1. Artefacts Part 2. Ecofacts Wessex Archaeology Report No. 23 Wessex Archaeology 2009 Published 2009 by Wessex Archaeology Ltd Portway House, Old Sarum Park, Salisbury, SP4 6EB http://www.wessexarch.co.uk Copyright © 2009 Wessex Archaeology Ltd All rights reserved ISBN 978-1-874350-49-1 Project website http://www.wessexarch.co.uk/projects/cambridgeshire/cambourne WA reports web pages http://www.wessexarch.co.uk/projects/cambridgeshire/cambourne/reports ii Contents Web pdf 1 Contents and Concordance of sites and summary details of archive ................................ iii Part 1. Artefacts 2 Prehistoric pottery, by Matt Leivers.....................................................................................1 2 Late Iron Age pottery, by Grace Perpetua Jones................................................................11 2 Romano-British pottery, by Rachael Seager Smith ...........................................................14 -

A New Species and New Genus of Clausiliidae (Gastropoda: Stylommatophora) from South-Eastern Hubei, China
Folia Malacol. 29(1): 38–42 https://doi.org/10.12657/folmal.029.004 A NEW SPECIES AND NEW GENUS OF CLAUSILIIDAE (GASTROPODA: STYLOMMATOPHORA) FROM SOUTH-EASTERN HUBEI, CHINA ZHE-YU CHEN1*, KAI-CHEN OUYANG2 1School of Life Sciences, Nanjing University, China (e-mail: [email protected]); https://orcid.org/0000-0002-4150-8906 2College of Horticulture & Forestry Science, Huazhong Agricultural University, China *corresponding author ABSTRACT: A new clausiliid species, in a newly proposed genus, Probosciphaedusa mulini gen. et sp. nov. is described from south-eastern Hubei, China. The new taxon is characterised by having thick and cylindrical apical whorls, a strongly expanded lamella inferior and a lamella subcolumellaris that together form a tubular structure at the base of the peristome, and a dorsal lunella connected to both the upper and the lower palatal plicae. Illustrations of the new species are provided. KEY WORDS: new species, new genus, systematics, Phaedusinae, central China INTRODUCTION In the past decades, quite a few authors have con- south-eastern Hubei, which is rarely visited by mala- ducted research on the systematics of the Chinese cologists or collectors, and collected some terrestrial Clausiliidae. Their research hotspots were mostly molluscs. Among them, a clausiliid was identified located in southern China, namely the provinces as a new genus and new species, and its respective Sichuan, Chongqing, Guizhou, Yunnan, Guangxi descriptions and illustrations are presented herein. and parts of Guangdong and Hubei, which have a Although some molecular phylogenetic studies have rich malacofauna (GREGO & SZEKERES 2011, 2017, focused on the Phaedusinae in East Asia (MOTOCHIN 2019, 2020, HUNYADI & SZEKERES 2016, NORDSIECK et al. -
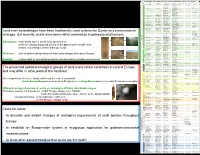
Ecological Groups of Snails – Use and Perspectives
The subdivision of all central European Holocene and Late Glacial land snail species to ecological groups ecological Glacial Early Holocene Middle Holocene Late Holocene (sensu Walker at al 2012) modern immigrants comment group Acanthinula aculeata Acanthinula aculeata Acanthinula aculeata Acanthinula aculeata Acicula parcelineata Acicula parcelineata Aegopinella epipedostoma one sites Aegopinella nitens Aegopinella nitens Aegopinella nitidula Aegopinella nitidula few sites Aegopinella pura Aegopinella pura Aegopinella pura Aegopinella pura Aegopis verticillus Ecological groups of snails Argna bielzi Argna bielzi Bulgarica cana Bulgarica cana Carpathica calophana Carpathica calophana one site; undated Causa holosericea Causa holosericea Clausilia bidentata no fossil data Clausilia cruciata Clausilia cruciata Clausilia cruciata – use and perspectives Cochlodina laminata Cochlodina laminata Cochlodina laminata Cochlodina laminata Cochlodina orthostoma Cochlodina orthostoma Cochlodina orthostoma Cochlodina orthostoma Daudebardia brevipes Daudebardia brevipes Daudebardia rufa Daudebardia rufa Daudebardia rufa Daudebardia rufa Discus perspectivus Discus perspectivus Discus perspectivus 1 2 1 1 ) Lucie Juřičková , Michal Horsák , Jitka Horáčková and Vojen Ložek Discus ruderatus Discus ruderatus Discus ruderatus Discus ruderatus Ena montana Ena montana Ena montana Ena montana forest Eucobresia nivalis Eucobresia nivalis Eucobresia nivalis Faustina faustina Faustina faustina Faustina faustina Faustina faustina Faustina rossmaessleri Faustina -

Presencia De Balea Heydeni Von Maltzan, 1881 (Gastropoda
Spira 6 (2016) 91–93 http://www.molluscat.com/spira.html Presencia de Balea heydeni von Maltzan, 1881 (Gastropoda: Clausiliidae) en Cantabria Jesús Ruiz Cobo1 & Sergio Quiñonero Salgado2,* 1Grupo de Espeleología e Investigaciones Subterráneas CarballoRaba, c/ Alcalde Arche s/n, 39600 Muriedas, Cantabria, Spain; 2Associació Catalana de Malacologia, Museu Blau, Plaça Leonardo da Vinci 45, 08019 Barcelona, Spain. Rebut el 5 de juliol de 2016 Acceptat el 2 d’octubre de 2016 © Associació Catalana de Malacologia (2016) Balea heydeni von Maltzan, 1881 es un molusco gasterópodo guignat, 1857 como un sinónimo de B. heydeni que tendría priori terrestre, perteneciente a la familia Clausiliidae, distribuido por dad. Sin embargo, por las razones aducidas por Gittenberger (2010) y buena parte del suroeste de Europa (Cadevall & Orozco, 2016). En Bank (2011), consideramos que el nombre correcto es Balea lucifuga España se conoce su presencia en Galicia y Asturias (Gittenberger Gray, 1824 (con distinta autoría), y que éste debe considerarse un et al., 2006; MartinezOrtí, 2006; Cadevall & Orozco, 2016). Aunque sinónimo posterior de Balea perversa. en Cantabria se ha citado Balea perversa (Linnaeus, 1758) (Altonaga Damos a conocer aquí la presencia de B. heydeni en las siguien et al., 1994; Cadevall & Orozco, 2016), a pesar de disponerse de un tes 18 localidades de Cantabria (Figuras 1–2). En todas ellas se en buen número de muestreos distribuidos por Cantabria, no hemos lo contraron conchas vacías en buen estado de conservación. Son las calizado esta especie. Todos los ejemplares del género Balea J.E Gray, siguientes, ordenadas aproximadamente de oeste a este: 1824 recolectados corresponden a B. -

Husbandry of the Carnivorous Land Snail, Powelliphanta Augusta (Gastropoda: Pulmonata: Rhytdidae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by ResearchArchive at Victoria University of Wellington Husbandry of the Carnivorous Land Snail, Powelliphanta augusta (Gastropoda: Pulmonata: Rhytdidae) By Thomas Edward Allan A thesis submitted to the Victoria University of Wellington in fulfillment of the requirements for the degree of Master of Science in Ecological Restoration Victoria University of Wellington 2010 1 Abstract Key aspects of the captive husbandry of Powelliphanta augusta, a newly-described New Zealand land snail are investigated: how they should be managed and fed to provide individuals for release, and how a long-term captive population can be maintained as an insurance against extinction in the wild. This project arises from almost all members of this species having been brought into captivity due to their displacement in the wild by an opencast coalmine. Powelliphanta (F: Rhytididae) is a genus of endemic carnivorous snails, which includes 10 species, 27 subspecies and numerous undescribed taxa. As well as its diversity, Powelliphanta is renowned for the large size of its members (up to 90mm diameter) and their attractively-patterned shells. Most taxa are threatened due to habitat loss and predation by introduced mammalian predators. The study commences with a literature review to refine husbandry methods and to assess requirements for captive breeding of snails. From this review investigations are made into stocking densities, substrate, reproductive biology, body condition and growth of the P. augusta captive population. To determine an appropriate stocking density for P. augusta groups of six snails were kept at two densities; with either 720cm2, or 1440cm2 per group. -

Plicuteria Lubomirski (Œlósarski, 1881)
Folia Malacol. 21(2): 91–97 http://dx.doi.org/10.12657/folmal.021.010 PLICUTERIA LUBOMIRSKI (ŒLÓSARSKI, 1881) (GASTROPODA: PULMONATA: HYGROMIIDAE), A FORGOTTEN ELEMENT OF THE ROMANIAN MOLLUSC FAUNA, WITH NOTES ON THE CORRECT SPELLING OF ITS NAME 1,* 2 3 4 BARNA PÁLL-GERGELY ,ROLAND FARKAS ,TAMÁS DELI ,FRANCISCO WELTER-SCHULTES 1 Department of Biology, Shinshu University, Matsumoto 390-8621, Japan (e-mail: [email protected]) 2 Aggtelek National Park Directorate, Tengerszem oldal 1, H-3758 Jósvafõ, Hungary (e-mail: [email protected]) 3 Békés Megyei Múzeumok Igazgatósága, Gyulai u 1., H-5600 Békéscsaba, Hungary (e-mail: [email protected]) 4 Zoologisches Institut, Berliner Strasse 28, 37073 Göttingen, Germany (e-mail: [email protected]) * corresponding author ABSTRACT: In this paper two new localities of the hygromiid land snail Plicuteria lubomirski (Œlósarski, 1881) are reported from Romania (Suceava and Harghita Counties). Its presence at the Lacu Roºu (Gyilkos-tó) area rep- resents the southeasternmost occurrence of the species. The only sample of P. lubomirski hitherto reported from Romania (leg. JICKELI in 1888 at Borsec Bai) seems to be lost from museum collections. The northern Carpathian distributional type is suggested for the species. The nomenclatural problem regarding the spelling of the specific name (i.e. lubomirskii or lubomirski) is discussed. The original, but less frequently used spelling (lubomirski) is suggested based on our understanding of the regulations of the ICZN. KEY WORDS: Carpathian species, faunistics, biogeography, Trochulus, anatomy INTRODUCTION Plicuteria lubomirski (Œlósarski, 1881) (often referred Umgebung des Bades Borszék” (=around Borsec Bai). to as Trochulus) is a Carpathian species reported from This record was later mentioned by SOÓS (1943) and Austria (KLEMM 1973, but also see REISCHÜTZ & GROSSU (1983).