Valeant, the Serial Pharma Dealmaker Cuts Again
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Faculty Disclosure
Faculty Disclosure In accordance with the ACCME Standards for Commercial Support, course directors, planning committees, faculty and all others in control of the educational content of the CME activity must disclose all relevant financial relationships with any commercial interest that they or their spouse/partner may have had within the past 12 months. If an individual refuses to disclose relevant financial relationships, they will be disqualified from being a part of the planning and implementation of this CME activity. Owners and/or employees of a commercial interest with business lines or products relating to the content of the CME activity will not be permitted to participate in the planning or execution of any accredited activity. Nature of Relevant Financial Relationship Last Name Commercial Interest What Was Received For What Role AbbVie, Allergan/ Tobira Therapeutics Inc, Gilead Research Grant Research Balart Sciences Inc, Pfizer, Salix Pharmaceuticals AbbVie, Merck Honorarium Advisory Board Bau None N/A N/A Benz None N/A N/A AbbVie, Arbutus Biopharma, Dieterich Gilead Sciences, Inc., Bristol- Research Grant Consultant Myers Squibb, Merck Bayer HealthCare Pharmaceuticals, Gilead Sciences Honorarium Speaking, Consultant Inc. Bristol-Myers Squibb, Gilead Speaking, Advisory Sciences, Inc, Salix Honorarium Frenette Board Pharmaceuticals, Inc, Merck Intercept Pharmaceuticals Honorarium Advisor Conatus Pharmaceuticals Inc Honorarium Consulting Principle Investigator, Research Grant, Han Gilead Sciences, -

Manufacturers and Wholesalers Street
Nevada AB128 Code of Conduct Compliant Companies Manufacturers and Wholesalers Street City ST Zip 10 Edison Street LLC 13 Edison Street LLC Abbott Diabetes Care Division Abbott Diagnostic Division Abbott Electrophysiology (including Kalila Medical 2- 2016)) Abbott Laboratories 100 Abbott Park Road, Dept. EC10, Bldg. APGA-2 Abbott Park IL 60064 Abbott Medical Optics Abbott Molecular Division Abbott Nutrition Products Division Abbott Vascular Division (includes Tendyne 9-2015) AbbVie, Inc. 1 N. Waukegan Road North Chicago IL 60064 Acadia Phamaceuticals 3611 Valley Centre Drive, Suite 300 San Diego CA 92130 Accelero Health Partners, LLC Acclarent, Inc. 1525-B O'Brien Dr. Menlo Park CA 94025 Accuri Cyometers, Inc. Ace Surgical Supply, Inc. 1034 Pearl St. Brockton MA 02301 Acorda Therapeutics, Inc. 420 Sawmill River Road Ardsley NY 10532 AcriVet, Inc. Actavis W.C. Holding, Inc. Morris Corporate Center III, 400 Interpace Parkway Parsippany NJ 07054 Actavis , Inc. Actelion Pharmaceuticals US, Inc. 5000 Shoreline Court, Suite 200 S. San Francisco CA 94080 Activis 400 Interpace parkway Parsippany NJ 07054 A-Dec, Inc. 2601 Crestview Dr. Newberg OR 97132 Advanced Respiratory, Inc. Advanced Sterilization Products 33 Technology Drive Irvine CA 92618 Advanced Vision Research, Inc., dba Akorn Consumer Health Aegerion Pharmaceuticals, Inc. 101 Main Street, Suite 1850 Cambridge MA 02142 Aesculap Implant Systems, Inc. Aesculap, Inc. 3773 Corporate Parkway Center Valley PA 18034 Aesthera Corporation Afaxys, Inc. PO Box 20158 Charleston SC 29413 AGMS, Inc. Akorn (New Jersey) Inc. Page 1 of 23 Pages 2/15/2017 Nevada AB128 Code of Conduct Compliant Companies Akorn AG (formerly Excelvision AG) Akorn Animal Health, Inc. -

Salix Pharmaceuticals, Inc. 2007 Annual Report and Form 10-K
2007 Annual Report and Form 10-K Advancing Treatment in GastroenterologyTM Corporate Mission Statement Salix is committed to being the leading U.S. specialty pharmaceutical Company licensing, developing and marketing innovative products to health care professionals to prevent or treat gastrointestinal disorders in patients while providing rewarding opportunities for our employees and creating exceptional value for our stockholders. To Our Stockholders Despite the December 28, 2007 approval of three generic delivery of mesalamine, or 5-ASA, beginning in the small balsalazide products by the Office of Generic Drugs, Salix bowel and continuing throughout the colon. Wilmington succeeded in making substantial advances in its business Pharmaceuticals, which licensed metoclopramide-ZYDIS to during 2007. From a product development standpoint we us, is moving forward in seeking FDA approval to market made impressive strides toward accessing both the multi- this fast-dissolving formulation. At this time, Wilmington is billion dollar irritable bowel syndrome market as well as targeting a fourth quarter 2008 approval. We believe that the hepatic encephalopathy market. We also progressed in our specialized sales force is positioned to effectively our effort to expand our presence in the inflammatory commercialize this patient-friendly formulation of this bowel disease market. On the marketing and sales side, widely-prescribed agent, if and when approved. we grew OSMOPREP® and MOVIPREP® to command a 25% We expect the product development success share of the prescription bowel cleansing market and we achieved during 2007 to be followed by commercial continued to grow XIFAXAN. On the business development success during 2008, as we anticipate receiving responses front we broadened our portfolio with the acquisitions of from the Food and Drug Administration during 2008 PEPCID OS® and metoclopramide-ZYDIS®. -

Spec Pharma M&A Transaction Multiples
SECTOR REPORT FOURTH QUARTER 2016 Disclaimer All information set forth in this report (the “Overview”) has been synthesized by Bourne Capital Partners, L.L.C. (“BP”) or was obtained from publicly available sources. BP makes no express or implied representation or warranty as to the accuracy or completeness of the information contained herein. BP expressly disclaims any and all liability that may be based on all information set forth in the Overview, errors therein, or omissions therefrom. This Overview includes certain statements, estimates and projections provided by BP with respect to anticipated future performance. Such statements, estimates and projections reflect various assumptions made by BP concerning anticipated results, which reflect significant subjective judgments made by BP and as a result, may or may not prove to be correct. There can be no assurance that such projected results are attainable or will be realized. No express or implied representations or warranties are made as to the accuracy of such statements, estimates or projections. In furnishing the Overview, BP does not undertake any obligation to provide the recipient with access to any additional information, to correct any inaccuracies that may become apparent or to update or otherwise revise this Overview. This Overview is not an offer to sell or a solicitation of an offer to purchase securities or to engage in any other transaction. BP is a North Carolina (USA) limited liability company doing business as Bourne Partners with divisions in Healthcare Merchant Banking, Alternative Assets, Management Consulting and Investment Banking. Investment Banking services are offered by Bourne Partners Securities, LLC, a registered broker dealer, Member FINRA and SIPC. -

Long-Term Efficacy and Safety of Etrasimod for Ulcerative Colitis
P0403 Long-term Efficacy and Safety of Etrasimod for Ulcerative Colitis: Results from the Open-label Extension of the OASIS Study Vermeire S1, Panés J2, Chiorean M3, Peyrin-Biroulet L4, Zhang J5, Sands BE6, Lazin K5, Cabell CH5, Naik SU5, Klassen P5, Sandborn WJ7 1Department of Gastroenterology, University Hospitals Leuven, Leuven, Belgium; 2Hospital Clinic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Spain; 3Division of Gastroenterology, Virginia Mason Medical Center, Seattle, WA, USA; 4Department of Gastroenterology, INSERM U954, Lorraine University, Vandoeuvre-lès-Nancy, France; 5Arena Pharmaceuticals, San Diego, CA, USA; 6Icahn School of Medicine at Mount Sinai, New York, NY, USA; 7University of California San Diego, La Jolla, CA, USA Introduction Results • Etrasimod is an oral, selective, sphingosine 1-phosphate receptors 1, Patient Disposition and Characteristics • Among patients achieving clinical response, clinical remission, or endoscopic Table 2. Summary of Adverse Events During the OLE in Patients Receiving Etrasimod 2 mg in the OLE (Safety Population) 4, and 5 (S1P1, S1P4, S1P5) modulator in development for treatment of • 118 patients (84% of DB completers) entered the OLE improvements at Week 12, treatment effects were maintained at EOT in 1 immune and inflammatory-mediated diseases — 112 patients (etrasimod safety population) received etrasimod 2 mg at the majority of patients (Figure 3) Etrasimod Etrasimod • The efficacy of etrasimod as induction therapy in adult patients with any point in the OLE Figure 3. Proportion of -
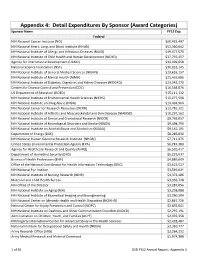
Appendix 4: Detail Expenditures by Sponsor (Award Categories) Sponsor Name FY12 Exp
Appendix 4: Detail Expenditures By Sponsor (Award Categories) Sponsor Name FY12 Exp. Federal NIH National Cancer Institute (NCI) $60,455,497 NIH National Heart, Lung, and Blood Institute (NHLBI) $52,360,642 NIH National Institute of Allergy and Infectious Diseases (NIAID) $49,477,570 NIH National Institute of Child Health and Human Development (NICHD) $37,797,457 Agency for International Development (USAID) $34,499,658 National Science Foundation (NSF) $30,033,145 NIH National Institute of General Medical Sciences (NIGMS) $29,655,157 NIH National Institute of Mental Health (NIMH) $25,435,686 NIH National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDKD) $23,042,270 Centers for Disease Control and Prevention (CDC) $16,568,876 US Department of Education (DOED) $15,111,132 NIH National Institute of Environmental Health Sciences (NIEHS) $15,077,598 NIH National Institute on Drug Abuse (NIDA) $14,494,964 NIH National Center for Research Resources (NCRR) $12,781,321 NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMSD) $10,297,162 NIH National Institute of Dental and Craniofacial Research (NIDCR) $9,769,057 NIH National Institute of Neurological Disorders and Stroke (NINDS) $9,498,790 NIH National Institute on Alcohol Abuse and Alcoholism (NIAAA) $9,161,191 Department of Energy (DOE) $8,285,830 NIH National Human Genome Research Institute (NHGRI) $7,711,975 United States Environmental Protection Agency (EPA) $6,787,380 Agency for Healthcare Research and Quality (AHRQ) $6,520,417 Department of Homeland -

Important Transaction and Tax Information Regarding the Exchange Transaction of Pharmaceutical Holdrs Trust Into Shares of Market Vectors Pharmaceutical Etf
IMPORTANT TRANSACTION AND TAX INFORMATION REGARDING THE EXCHANGE TRANSACTION OF PHARMACEUTICAL HOLDRS TRUST INTO SHARES OF MARKET VECTORS PHARMACEUTICAL ETF For electing owners (“Exchange Participants”) of Pharmaceutical HOLDRS Trust (“PPH HOLDRS”), units of PPH HOLDRS were exchanged on December 20, 2011 for shares of Market Vectors Pharmaceutical ETF. For each PPH HOLDR receipt validly tendered, Exchange Participants received 1 share of Market Vectors Pharmaceutical ETF. This exchange was effected through a series of transfers and rebalance transactions (sales transactions in certain securities underlying the PPH HOLDRS and purchase transactions in new securities for exchange into Market Vectors Pharmaceutical ETF). These transactions were authorized by the Exchange Participant and executed on their behalf. As a result of the rebalance transactions, part of this exchange may result in a taxable event and cost basis adjustments to the Exchange Participant. Provided is a breakdown of the exchange transactions and tax implications based on a ‘PPH HOLDR trading unit” (100 PPH HOLDRS receipts) 1. For each PPH HOLDR Trading Unit tendered, BNY Mellon Shareowner Services as Exchange Agent for the exchange offer, delivered the following securities per PPH HOLDR Trading Unit to BNY ConvergEx Execution Solutions LLC (“ConvergEx”), acting in its capacity transition manager in connection with the exchange offer. The total value of these securities transferred per PPH HOLDR Trading Unit, based on the December 20, 2011 market close, was $7,191.63. -

FY06 Pharmaceutical Marketing Disclosures Report
Pharmaceutical Marketing Disclosures For the period July 1, 2005 to June 30, 2006 Report of Vermont Attorney General William H. Sorrell June 26, 2007 Contact: Julie Brill Assistant Attorney General (802) 828-5479 Pharmaceutical Marketing Disclosures: Report of Vermont Attorney General William H. Sorrell on Payments to Physicians June 26, 2007 Index Page: I. Executive Summary 3 II. Description of Vermont’s Payment Disclosure Law 4 III. Amendments to Prior Pharmaceutical Marketing Disclosure Reports filed by the Vermont Attorney General's Office 5 IV. Summary of Pharmaceutical Marketing Expenditures 6 1. Total Payments of Each Pharmaceutical Manufacturer 6 2. Payments of all Manufacturers Organized by Recipient Type 7 3. Payments by Nature of Expenditure 10 4. Payments by Purpose of Expenditure 10 5. Trade Secret Declarations 11 V. Enforcement Actions 12 Appendix: Tab 1: 33 V.S.A. §2005 13 Tab 2: FY 06 Tables 17 Tab 3: FY 05 Tables as Revised 25 Tab 4: FY 04 Tables as Revised 34 Tab 5: FY 03 Tables as Revised 43 2 Pharmaceutical Marketing Disclosures: Report of Vermont Attorney General William H. Sorrell on Payments to Physicians June 26, 2007 I. Executive Summary This is the fourth report of Vermont Attorney General William H. Sorrell on Pharmaceutical Marketing Disclosures. It is based upon disclosures pertaining to payments made during the period July 1, 2005, through June 30, 2006 (FY 06) by pharmaceutical marketers, of the amount of money the companies paid during the past fiscal year on consulting and speaker fees, travel expenses, gifts, and other payments to physicians, hospitals, universities and others authorized to prescribe or dispense pharmaceutical products. -

Appendix 1: Detail Awards by Sponsor
Appendix 1: Detail Awards By Sponsor Sponsor Name FY12 Award Federal National Cancer Institute $50,418,830 National Institute of Allergy and Infectious Diseases $46,180,948 US Agency for International Development $37,208,131 National Science Foundation - Research (NSF) $32,365,368 National Institute of Child Health and Human Development (NICHD) $31,370,905 National Heart Lung and Blood Institute (NHLBI) $30,091,872 National Institute of Diabetes, Digestive and Kidney Diseases $24,989,100 National Institute of General Medicine Science $22,515,093 National Institute of Mental Health-NIH $19,346,563 National Heart Lung and Blood Institute $15,390,835 US Department of Education $15,016,549 National Institute on Drug Abuse $12,872,528 NIH National Center for Advancing Translational Sciences (NCATS) $12,546,906 National Institute of Environmental Health Sciences $12,130,116 National Institute on Alcohol Abuse and Alcoholism $11,320,987 National Institute of Arthritis Musculoskeletal Skin Disease $10,112,508 Centers for Disease Control $9,028,629 National Institute of Dental and Craniofacial Research $8,401,136 National Institute of Neurologic Disorders and Stroke $8,378,473 Bureau of Health Professions $8,085,816 US Department of Homeland Security $5,699,790 US Department of Energy $5,266,325 Centers for Disease Control and Prevention (CDC) $4,615,173 Agency for Healthcare Research and Quality $4,547,997 US Army Medical Research $4,534,927 NIH National Heart, Lung, and Blood Institute (NHLBI) $4,425,558 US Environmental Protection Agency - -

Marketer1 Year of Initial Investment Investment
YEAR OF INITIAL INVESTMENT COUNTERPARTY PRODUCT(S) MARKETER1 INVESTMENT VEHICLE Artes Medical, Inc. Bellafill, Puregraft, HD PRP Artes Medical, Inc. 2008 HCR 1 Vertex Pharmaceuticals, Inc. Lexiva ViiV Healthcare UK Limited 2008 HCR 1 Dyax Corp. Phage display platform Multiple 2008 HCR 1, SMA Undisclosed Sanctura, Sanctura XR Allergan, Inc. 2008 HCR 1 Shore Therapuetics, Inc. Fenoglide Sciele Pharma, Inc. 2008 HCR 1 Undisclosed Oracea, Sanctura XR Galderma S.A. / Allergan, Inc. 2008 HCR 1, HCR 2 AEterna Zentaris, Inc. Cetrotide Merck Serono S.A. 2008 HCR 1 NeurogesX, Inc. Qutenza Astellas Pharma Inc. 2010 HCR 1 Undisclosed Undisclosed vaccine Undisclosed 2010 HCR 1 Undisclosed Myozyme Genzyme Corp. / Sanofi-Aventis U.S. LLC 2011 HCR 1 Inventor (undisclosed) Krystexxa Savient Pharmaceuticals, Inc. 2011 HCR 1 Undisclosed Undisclosed vaccine Undisclosed 2010 HCR 2 Zogenix, Inc. Sumavel Dosepro, Zohydro Zogenix, Inc. 2011 HCR 2 AcuFocus, Inc. Kamra Inlay, IOL AcuFocus, Inc. 2011 HCR 2 Inventor Cervarix GlaxoSmithKline plc 2011 HCR 2 Stereotaxis, Inc. RMT Ablation Catheters Biosense Webster, Inc. 2011 HCR 2 Helomics Corporation ChemoFx, GeneFx Lung, GeneFx Colon, BioSpeciFx Helomics Corporation 2012 HCR 2 Raptor Pharmacuetical Corp. Procysbi Raptor Pharmacuetical Corp. 2012 HCR 2, SMA Undisclosed Undisclosed Undisclosed 2012 HCR 2 Nuron Biotech, Inc. Meningitec, HibTITER Nuron Biotech, Inc. 2012 HCR 2 Medigene AG Eligard Astellas Pharma Inc. 2012 HCR 2 Undisclosed Lyrica Pfizer, Inc. 2012 HCR 2, SMA Ironwood Pharmaceuticals, Inc. Linzess Allergan, Inc. 2012 SMA TearScience, Inc. LipiView, LipiFlow TearScience, Inc. 2013 HCR 2 Undisclosed Inavir Daiichi Sankyo Company, Limited 2013 HCR 2 Undisclosed Benlysta GlaxoSmithKline plc 2013 HCR 2 Suneva Medical, Inc. -

Manufacturers and Wholesalers Street City ST Zip Abbott Diabetes Care Division Abbott Diagnostic Division Abbott Laboratories 100 Abbott Park Road, Dept
2018 AB128 Code of Conduct Compliant Companies report Manufacturers and Wholesalers Street City ST Zip Abbott Diabetes Care Division Abbott Diagnostic Division Abbott Laboratories 100 Abbott Park Road, Dept. EC10, Bldg. APGA-2 Abbott Park IL 60064 Abbott Molecular Division Abbott Nutrition Products Division Abbott Vascular Division AbbVie, Inc. 1 N. Waukegan Road North Chicago IL 60064 Acadia Phamaceuticals 3611 Valley Centre Drive, Suite 300 San Diego CA 92130 Accelero Health Partners, LLC Acclarent, Inc. 1525-B O'Brien Dr. Menlo Park CA 94025 Accutron Inc. Ace Surgical Supply, Inc. 1034 Pearl St. Brockton MA 02301 Acell, Inc. 6640 Eli Whitney Drive, Suite 200 Columbia MD 21046 Aclaris Therapeutics, Inc. 640 Lee Road, Suite 200 Wayne PA 19087 Acorda Therapeutics, Inc. 420 Sawmill River Road Ardsley NY 10532 AcriVet Inc. Actelion Pharmaceuticals US, Inc. 5000 Shoreline Court, Suite 200 S. San Francisco CA 94080 Adamas Pharmaceuticals, Inc. 1900 Powell St., Suite 750 Emeryville CA 94608 A-Dec, Inc. 2601 Crestview Dr. Newberg OR 97132 Advanced Respiratory, Inc. Advanced Sterilization Products Services, Inc. Aegerion Pharmaceuticals, Inc. One Main Street, Suite 800 Cambridge MA 02142 Aerie Pharmaceuticals, Inc. 2030 Main St., Suite 1400 Irvine CA 92614 Aesculap Implant Systems, Inc. Aesculap, Inc. 3773 Corporate Parkway Center Valley PA 18034 Aesthera Corporation Afaxys, Inc. PO Box 20158 Charleston SC 29413 AGMS Inc. Akrimax Pharmaceuticals, LLC 11 Commerce Drive, 1st Floor Cranford NJ 07016 Alamo Pharma Services, Inc. Alcon Laboratories, Inc. 6201 South Freeway Fort Worth TX 76134 Alden Optical Laboratories, Inc. Aldenex Vision LLC Alimera Sciences, Inc. 6120 Windward Parkway, Suite 290 Alpharetta GA 30005 Alkermes, Inc. -

Speciality Players Define M&A As Big Pharma Seeks Focus
May 26, 2015 Speciality players define M&A as big pharma seeks focus Jonathan Gardner Forget megamergers. For half a decade the daring deals in the pharma space have been made primarily by the speciality players. Endo International’s move on Par Pharmaceutical last week shows how this peer group has defined M&A since 2009, when Pfizer and Merck & Co made the last major acquisitions of companies regarded as traditional big pharmas, those of Wyeth and Schering-Plough respectively. One company alone, Actavis, has been responsible for nearly a third of the value of the top 25 deals in this time, transforming itself from a respectably sized generics company into the world’s second-biggest seller of skin and eye disease drugs (see table below). Tax pressure To be sure, big pharma companies have been active acquirers, but their activities have been confined primarily to achievable bolt-on transactions to bring in interesting developmental assets or promising business lines, not the megamergers that tend to grab headlines. Two deals proposed and then abandoned last year might have changed this picture substantially and moved nearly $150bn onto the side of big pharma. AstraZeneca successfully fought off Pfizer, however, and AbbVie had a near-miss with its takeout of Shire – scuttled because of changes to US tax rules. In fact it was speciality companies rather than pharma giants that wrung most of the value from tax deals before laws making inversions much less appealing were enacted. Before Actavis became the world’s 19th biggest seller of prescription drugs, and on its way to a top 10 ranking, it was known as Watson Pharmaceuticals.