Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clearing the Smoke on Cannabis: Regular Use and Cognitive Functioning
6 Clearing the Smoke on Cannabis Regular Use and Cognitive Functioning Robert Gabrys, Ph.D., Research and Policy Analyst, CCSA Amy Porath, Ph.D., Director, Research, CCSA Key Points • Regular use refers to weekly or more frequent cannabis use over a period of months to years. Regular cannabis use is associated with mild cognitive difficulties, which are typically not apparent following about one month of abstinence. Heavy (daily) and long-term cannabis use is related to more This is the first in a series of reports noticeable cognitive impairment. that reviews the effects of cannabis • Cannabis use beginning prior to the age of 16 or 17 is one of the strongest use on various aspects of human predictors of cognitive impairment. However, it is unclear which comes functioning and development. This first — whether cognitive impairment leads to early onset cannabis use or report on the effects of chronic whether beginning cannabis use early in life causes a progressive decline in cannabis use on cognitive functioning cognitive abilities. provides an update of a previous report • Regular cannabis use is associated with altered brain structure and function. with new research findings that validate Once again, it is currently unclear whether chronic cannabis exposure and extend our current understanding directly leads to brain changes or whether differences in brain structure of this issue. Other reports in this precede the onset of chronic cannabis use. series address the link between chronic • Individuals with reduced executive function and maladaptive (risky and cannabis use and mental health, the impulsive) decision making are more likely to develop problematic cannabis use and cannabis use disorder. -

Cannabis – a Complex and Rapidly Evolving Landscape
CANNABIS – A COMPLEX AND RAPIDLY EVOLVING LANDSCAPE Abstract ABOUT THE AUTHOR The humble Cannabis sativa plant, cultivated for millennia for its psychoactive properties and more, is today considered one of the most controversial and complex plants in the world. Starting in the early to mid-20th century, much of its use became recreational, but by the early 1970s discoveries began to emerge around its potential medical efficacies. This article will discuss current knowledge of how cannabis engages with the brain and the endocannabinoid system (ECS) and provide an overview of the new market landscapes brought about by changes in governing laws Dr. Georgiana Willwerth-Pascutiu and regulations, which are affecting usage by our current and potential [email protected] customers. It will also explore the additional hazards, concerns, and Georgiana Willwerth-Pascutiu is Vice President, Global Medical Director considerations of cannabis use in countries where it remains illegal. at RGA. She is board certified in Insurance Medicine by the American Introduction Academy of Insurance Medicine (AAIM) and specialized in internal medicine, Naturally occurring psychoactive substances have been part of human life nephrology and ultrasonography. for millennia. One of the most frequently utilized plant sources of these Dr. Willwerth-Pascutiu is also a past substances, Cannabis sativa, is also the best-known worldwide. For the president of the Canadian Life Insurance Medical Officers Association (CLIMOA) past half-century, scientific and medical interest in its many compounds, and currently chairs its scientific known as cannabinoids, has been increasing. Today, the two best-known, committee. She is a frequent presenter and has contributed several articles to delta-9 tetrahydrocannabinol (THC), its psychoactive chemical, and insurance industry publications. -

Medical Cannabis Q&A
Medical Cannabis Q&A 1. What is medical cannabis? The term “medical cannabis” is used to describe products derived from the whole cannabis plant or its extracts containing a variety of active cannabinoids and terpenes, which patients take for medical reasons, after interacting with and obtaining authorization from their health care practitioner. 2. What are the main active ingredients? The chemical ingredients of cannabis are called cannabinoids. The two main therapeutic ones are: THC:CBD a. Tetrahydrocannabinol (THC) is a partial agonist of CB1 and CB2 receptors. It is psychoactive and produces the euphoric effect. Each cannabis product will contain THC and CBD, however b. Cannabidiol (CBD) has a weak affinity for CB1 and CB2 receptors and appears the THC: CBD ratio will differ to exert its activity by enhancing the positive effects of the body’s endogenous depending on the product. cannabinoids. 3. Why do patients take it? Medical cannabis may be used to alleviate symptoms for a variety of conditions. It has most commonly been used in neuropathic pain and other chronic pain conditions. There is limited, but developing clinical evidence surrounding its safety and efficacy, and it does not currently have an approved Health Canada indication. 4. How do patients take it? Cannabis can be smoked, vaporized, taken orally, sublingually, topically or rectally. Different routes of administration will result in different pharmacokinetic and pharmacodynamic properties of the drug. 5. Is it possible to develop dependence on medical cannabis? Yes, abrupt discontinuation after long-term use may result in withdrawal symptoms. Additionally, chronic use may result in psychological dependence. -

A Review on Cannabis Sativa: Its Compounds and Their Effects
Int. J. Pharm. Sci. Rev. Res., 53(2), November - December 2018; Article No. 12, Pages: 59-63 ISSN 0976 – 044X Review Article A Review on Cannabis sativa: Its Compounds and Their Effects Ranju Rajput, *Dr. Krishan Kumar Department of Food and Biotechnology, Jayoti Vidyapeeth Women’s University, Jaipur, Rajasthan, India. *Corresponding author’s E-mail: [email protected] Received: 01-11-2018; Revised: 25-11-2018; Accepted: 10-12-2018. ABSTRACT Our society often considered the use of cannabis is an under-reported activity. Cannabis is used to relieve neuropathic and chronic pain. Cannabis, produced from the Cannabis sativa plant, have been used in three forms: herbal cannabis, the dried leaves and flowering tops The resin of the cannabis is the pressed secretions of the plant, known as ‘hashish’ or ‘charash. Cannabis sativa is an herbaceous species originated from Central Asia. It has been used in medicine and as a source of textile fiber since ancient times. The cannabis sativa is a fast growing plant attracted the people’s interest because of its multi-purpose applications. It is a rich source of photochemical, cellulose and woody fibers. The more interest is also due to its metabolites which show potent bioactivities on human health. In this review, the phytochemicals is discussed by putting a special emphasis on molecules including cannabinoids, terpenes and phenolic compounds. Cannabinoids are represented as the most studied group of compounds, because of their wide range of pharmaceutical effects in humans, including psychotropic activities. This article aims to update the current knowledge and evidence of using cannabis and its derivatives with a view to the sociolegal context and perspectives for future research. -

Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System
International Journal of Molecular Sciences Review Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System Shenglong Zou and Ujendra Kumar * Faculty of Pharmaceutical Sciences, The University of British Columbia, Vancouver, BC V6T 1Z4, Canada; [email protected] * Correspondence: [email protected]; Tel.: +1-604-827-3660; Fax: +1-604-822-3035 Received: 9 February 2018; Accepted: 11 March 2018; Published: 13 March 2018 Abstract: The biological effects of cannabinoids, the major constituents of the ancient medicinal plant Cannabis sativa (marijuana) are mediated by two members of the G-protein coupled receptor family, cannabinoid receptors 1 (CB1R) and 2. The CB1R is the prominent subtype in the central nervous system (CNS) and has drawn great attention as a potential therapeutic avenue in several pathological conditions, including neuropsychological disorders and neurodegenerative diseases. Furthermore, cannabinoids also modulate signal transduction pathways and exert profound effects at peripheral sites. Although cannabinoids have therapeutic potential, their psychoactive effects have largely limited their use in clinical practice. In this review, we briefly summarized our knowledge of cannabinoids and the endocannabinoid system, focusing on the CB1R and the CNS, with emphasis on recent breakthroughs in the field. We aim to define several potential roles of cannabinoid receptors in the modulation of signaling pathways and in association with several pathophysiological conditions. We believe that the therapeutic significance of cannabinoids is masked by the adverse effects and here alternative strategies are discussed to take therapeutic advantage of cannabinoids. Keywords: cannabinoid; endocannabinoid; receptor; signaling; central nervous system 1. Introduction The plant Cannabis sativa, better known as marijuana, has long been used for medical purpose throughout human history. -

N-Acyl-Dopamines: Novel Synthetic CB1 Cannabinoid-Receptor Ligands
Biochem. J. (2000) 351, 817–824 (Printed in Great Britain) 817 N-acyl-dopamines: novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo Tiziana BISOGNO*, Dominique MELCK*, Mikhail Yu. BOBROV†, Natalia M. GRETSKAYA†, Vladimir V. BEZUGLOV†, Luciano DE PETROCELLIS‡ and Vincenzo DI MARZO*1 *Istituto per la Chimica di Molecole di Interesse Biologico, C.N.R., Via Toiano 6, 80072 Arco Felice, Napoli, Italy, †Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, R. A. S., 16/10 Miklukho-Maklaya Str., 117871 Moscow GSP7, Russia, and ‡Istituto di Cibernetica, C.N.R., Via Toiano 6, 80072 Arco Felice, Napoli, Italy We reported previously that synthetic amides of polyunsaturated selectivity for the anandamide transporter over FAAH. AA-DA fatty acids with bioactive amines can result in substances that (0.1–10 µM) did not displace D1 and D2 dopamine-receptor interact with proteins of the endogenous cannabinoid system high-affinity ligands from rat brain membranes, thus suggesting (ECS). Here we synthesized a series of N-acyl-dopamines that this compound has little affinity for these receptors. AA-DA (NADAs) and studied their effects on the anandamide membrane was more potent and efficacious than anandamide as a CB" transporter, the anandamide amidohydrolase (fatty acid amide agonist, as assessed by measuring the stimulatory effect on intra- hydrolase, FAAH) and the two cannabinoid receptor subtypes, cellular Ca#+ mobilization in undifferentiated N18TG2 neuro- CB" and CB#. NADAs competitively inhibited FAAH from blastoma cells. This effect of AA-DA was counteracted by the l µ N18TG2 cells (IC&! 19–100 M), as well as the binding of the CB" antagonist SR141716A. -

Cannabinoid As Potential Aromatase Inhibitor Through Molecular Modeling and Screening for Anti-Cancer Activity
Cannabinoid as Potential Aromatase Inhibitor Through Molecular Modeling and Screening for Anti-Cancer Activity Sudipta Baroi1, Achintya Saha2, Ritesh Bachar3 and Sitesh C Bachar4 1Department of Pharmaceutical Technology, Faculty of Pharmacy, University of Dhaka Dhaka-1000, Bangladesh 2Department of Chemical Technology, Pharmaceutical & Fine Chemical Technology Division University of Calcutta, India 3Department of Pharmacy, School of Science and Engineering, University of Information Technology and Sciences (UITS), Dhaka-1212, Bangladesh 4Department of Pharmacy, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh (Received: April 15, 2020; Accepted: June 2, 2020; Published (web): June 28, 2020) ABSTRACT: Inhibition of aromatase (CYTP450), a key enzyme in the estrogen biosynthesis, could result in regression of estrogen-dependent tumors and even prevent the promotion of breast cancer. The present research has been designed for searching a potent chemical moiety from natural sources to inhibit aromatase enzyme, the over- functionality of which causes the breast cancer. Cannabis sativa contains a very much promising group of cannabinoids with more than 66 compounds with reported anticancer property and for the search of a target specific potent aromatase inhibitor, 61 cannabinoids from C. sativa were selected. The Structures Data File (SDF) of these ligand molecules were subjected to docking studies at the binding site of aromatase X-ray crystallographic structure based on lower resolution of the protein crystal structure and higher docking accuracy, predicted by calculating the correlation between experimental activities and Glide dock scores and compared with the standard aromatase ligand androstenedione and aromatase inhibitor fadrozole with existing drug for breast cancer treatment. The best docked pose of each ligand was selected on the basis of the highest dock score related to the binding free energies of the internal dataset compounds as compared to their observed activities. -

15.04.610.270 - Marijuana/Cannabis Commercial Uses
15.04.610.270 - Marijuana/Cannabis Commercial Uses. Commercial Cannabis activities, including but not limited to cultivation, manufacturing, testing, distribution, and retail are subject to the standards and procedures of the Municipal Code, State Law, and the regulations set forth in these Zoning Regulations. A. Applicability. These standards apply to all establishments that are involved in any commercial cannabis activity. B. Definitions1 []. The following words or phrases, whenever used in this section, have the following definitions: 1. A-license. A State license issued for cannabis or cannabis products that are intended for adults 21 years of age and over and who do not possess physician's recommendations. 2. Attending Physician. An individual who possesses a license in good standing to practice medicine or osteopathy issued by the Medical Board of California or the Osteopathic Medical Board of California and who has taken responsibility for an aspect of the medical care, treatment, diagnosis, counseling, or referral of a patient and who has conducted a medical examination of that patient before recording in the patient's medical record the physician's assessment of whether the patient has a serious medical condition and whether the medical use of cannabis is appropriate. 3. Bureau of Cannabis Control ("the Bureau"). The bureau within the California Department of Consumer Affairs created to develop, administer and enforce comprehensive rules for medicinal and adult-use cannabis in California. The Bureau is responsible for the regulation and licensing of all commercial cannabis retail, distribution, testing, microbusinesses and temporary cannabis events in California. 4. California Department of Food and Agriculture — CalCannabis Cultivation Licensing ("the CDFA"). -

A Dissertation Entitled Uncovering Cannabinoid Signaling in C. Elegans
A Dissertation Entitled Uncovering Cannabinoid Signaling in C. elegans: A New Platform to Study the Effects of Medicinal Cannabis By Mitchell Duane Oakes Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Biology ________________________________________ Dr. Richard Komuniecki, Committee Chair _______________________________________ Dr. Bruce Bamber, Committee Member ________________________________________ Dr. Patricia Komuniecki, Committee Member ________________________________________ Dr. Robert Steven, Committee Member ________________________________________ Dr. Ajith Karunarathne, Committee Member ________________________________________ Dr. Jianyang Du, Committee Member ________________________________________ Dr. Amanda Bryant-Friedrich, Dean College of Graduate Studies The University of Toledo August 2018 Copyright 2018, Mitchell Duane Oakes This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Uncovering Cannabinoid Signaling in C. elegans: A New Platform to Study the Effects of Medical Cannabis By Mitchell Duane Oakes Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Biology The University of Toledo August 2018 Cannabis or marijuana, a popular recreational drug, alters sensory perception and exerts a range of medicinal benefits. The present study demonstrates that C. elegans exposed to -

Personal Use Cannabis Rules Special Adopted New Rules: N.J.A.C
NEW JERSEY CANNABIS REGULATORY COMMISSION Personal Use Cannabis Rules Special Adopted New Rules: N.J.A.C. 17:30 Adopted: August 19, 2021 by New Jersey Cannabis Regulatory Commission, Dianna Houenou, Chair. Filed: August 19, 2021 Authority: N.J.S.A. 24:6I-31 et seq. Effective Date: August 19, 2021 Expiration Date: August 19, 2022 This rule may be viewed or downloaded from the Commission’s website at nj.gov/cannabis. These rules are adopted pursuant to N.J.S.A. 24:6I-34(d)1a of the New Jersey Cannabis Regulatory, Enforcement Assistance, and Marketplace Modernization Act, N.J.S.A. 24:6I- 31 et seq., and became effective upon acceptance for filing by the Office of Administrative Law. The specially adopted new rules shall be effective for a period not to exceed one year from the date of filing of the new rules, that is, until August 19, 2022. The Commission has provided this special adoption to the Attorney General, State Treasurer, Commissioner of Health, and Commissioner of Banking and Insurance for a consultation period, after which the Commission anticipates filing a proposal to readopt these rules with amendments reflecting the results of that consultation. In accordance with N.J.S.A. 24:6I-34(d)1b the rules, as readopted, will become effective upon acceptance for filing by the Office of Administrative Law if filed on or before the expiration date of the rules published herein. The adopted amendments will be effective upon publication in the New Jersey Register. Federal Standards Analysis The Cannabis Regulatory, Enforcement Assistance, and Marketplace Modernization Act obliges the Cannabis Regulatory Commission to promulgate rules necessary or proper to enable it to carry out the Commission’s duties, functions, and powers with respect to overseeing the development, regulation, and enforcement of activities associated with the personal use of cannabis pursuant to P.L.2021, c.16. -
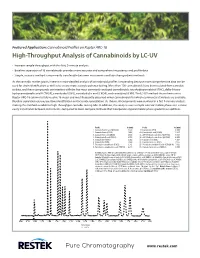
High-Throughput Analysis of Cannabinoids by LC-UV
Featured Application: Cannabinoid Profiles on Raptor ARC-18 High-Throughput Analysis of Cannabinoids by LC-UV • Increase sample throughput with this fast, 9-minute analysis. • Baseline separation of 16 cannabinoids provides more accurate and comprehensive potency and profile data. • Simple, isocratic method is more easily transferable between instruments and labs than gradient methods. As the cannabis market grows, interest in more detailed analysis of cannabinoid profiles is expanding because more comprehensive data can be used for strain identification as well as to ensure more accurate potency testing. More than 100 cannabinoids have been isolated from cannabis to date, and these compounds can interfere with the five most commonly analyzed cannabinoids: tetrahydrocannabinol (THC), delta-9-tetra- hydrocannabinolic acid A (THCA), cannabidiol (CBD), cannabidiolic acid (CBDA), and cannabinol (CBN). The LC-UV method shown here uses a Raptor ARC-18 column to fully resolve 16 major and most frequently observed minor cannabinoids for which commercial standards are available. Baseline separation ensures positive identification and accurate quantitation. As shown, all compounds were resolved in a fast 9-minute analysis, making this method suitable for high-throughput cannabis testing labs. In addition, this analysis uses a simple isocratic mobile phase so it is more easily transferable between instruments, compared to more complex methods that incorporate atypical mobile phase gradients or additives. Peaks tR (min) Peaks tR (min) 1. Cannabidivarinic acid (CBDVA) 1.877 9. Cannabinol (CBN) 4.609 2. Cannabidivarin (CBDV) 2.086 10. Cannabinolic acid (CBNA) 5.437 3. Cannabidiolic acid (CBDA) 2.592 11. Δ9-Tetrahydrocannabinol (Δ9-THC) 5.815 4. Cannabigerolic acid (CBGA) 2.750 12. -

CBD (Cannabidiol)
TRANSPORTATION RESEARCH BOARD Driving Toward the Truth - Dispelling the Myths About Cannabis Products February 10, 2021 @NASEMTRB #TRBwebinar PDH Certification The Transportation Research Board has met the standards and Information: requirements of the Registered Continuing Education Providers •1.5 Professional Development Program. Credit earned on completion Hour (PDH) – see follow-up of this program will be reported to email for instructions RCEP. A certificate of completion will •You must attend the entire be issued to participants that have registered and attended the entire webinar to be eligible to receive session. As such, it does not include PDH credits content that may be deemed or •Questions? Contact Reggie construed to be an approval or Gillum at [email protected] endorsement by RCEP. #TRBwebinar Learning Objectives 1. Identify impacts of the Farm Bill on use of THC and CBD products 2. Describe the toxicology of THC and CBD products 3. Discuss how THC and CBD products affect driving performance and crash risk #TRBwebinar TRB Standing Committee on Impairment in Transportation (ACS50) TRB Webinar: Driving Toward the Truth - Dispelling the Myths About Cannabis Products Dr. Barry K. Logan Executive Director, Center for Forensic Science Research and Education (CFSRE); Senior Vice President of Forensic Sciences, and Chief Scientist at NMS Labs Michelle Peace, Ph.D. Associate Professor and PI, Laboratory for Forensic Toxicology Research Department of Forensic Science, Virginia Commonwealth University Dr. Darrin Grondel Vice President,