Infection, Disease & Health
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BOOKLIST for ADN Program Spring Quarter (January 4, 2021)
BOOKLIST for ADN Program Spring Quarter (January 4, 2021) The following outlines the resources (textbooks, websites, media, etc.) utilized in each course. Required resources are necessary for academic success: you must obtain and study from them to succeed in a course as outlined in the course syllabus. Recommended resources provide material that will boost your academic performance in that course but are not always necessary for academic success. Faculty may or may not use recommended resources in class or during clinical. Please confer with course faculty prior to purchasing recommended resources. Use International Standard Book Numbers (ISBN) to identify the books below with any book vendor. Rented, used, and electronic formats of the resources may be available at a reduced cost to the student. A dagger (†) preceding a title indicates the resource is appropriate for renting. A keyboard symbol (⌨) preceding a title indicates the resource is available electronically/online. When renting a textbook, it may not include CDs, DVDs, or access codes for other protected electronic content, even if the book had those features when sold new. These features may be required for successful completion of the course in which the book is used. If you believe a textbook is supposed to include a disk or an unused access code, check before renting. Galen is not responsible for purchases made in error. Textbooks/resources may be used in multiple courses throughout the program. Students should verify this information via the booklist prior to purchasing/renting or returning/reselling. Galen encourages students to retain textbooks/resources as reference materials to assist throughout their nursing career. -

CURRICULUM VITAE Floyd L
CURRICULUM VITAE Floyd L. Wormley Jr., Ph.D. Office of Research and Graduate Studies Texas Christian University TCU Box 297024 Fort Worth, TX 76129 Phone: (817) 257-7104 ADDRESS: 7121 Axis Ct. Fort Worth, TX 76132 Tel: (817) 386-0645, E-mail: [email protected] ACADEMIC TRAINING: 2002-2005 Doctoral Fellowship, Infectious Diseases Duke University Medical Center, Durham, NC Interdisciplinary Research Training Program in AIDS Program in Microbial Pathogenesis of Cryptococcus neoformans Research Project: Study of host-pathogen interactions during Cryptococcus neoformans infection. Fellowship Advisor: John R. Perfect, M.D. 1998-2001 Doctor of Philosophy, Microbiology/Immunology Louisiana State University Health Sciences Center, New Orleans, LA Dissertation Title: Cell Mediated Immunity Against Experimental Candida albicans Vaginitis. Dissertation Advisor: Paul L. Fidel Jr., Ph.D. 1995-1998 Master of Science, Microbiology/Immunology Louisiana State University Health Sciences Center, New Orleans, LA Thesis Title: Evidence for a Unique CD4 Protein on Murine Vaginal CD4+ T Cells. Thesis Advisor: Paul L. Fidel Jr., Ph.D. 1990-1995 Bachelor of Science, Cellular and Molecular Biology Tulane University, New Orleans, LA. EMPLOYMENT: 2019-Present Associate Provost for Research and Dean of Graduate Studies, Texas Christian University, Fort Worth, TX Role and Responsibility – Promote the advancement of research within the institution and administer key compliance programs through which we promote the responsible conduct of research and integrity. I oversee all research policies and procedures, institutional research compliance committees, and research technology and innovation. I also oversee services and programs for those who seek financial support for their scholarly endeavors from inception of idea through submission and award of research/scholarly project. -

Role of Infection and Immunity in Bovine Perinatal Mortality: Part 2
animals Review Role of Infection and Immunity in Bovine Perinatal Mortality: Part 2. Fetomaternal Response to Infection and Novel Diagnostic Perspectives Paulina Jawor 1,* , John F. Mee 2 and Tadeusz Stefaniak 1 1 Department of Immunology, Pathophysiology and Veterinary Preventive Medicine, Wrocław University of Environmental and Life Sciences, 50-375 Wrocław, Poland; [email protected] 2 Animal and Bioscience Research Department, Teagasc, Moorepark Research Centre, P61 P302 Fermoy, County Cork, Ireland; [email protected] * Correspondence: [email protected] Simple Summary: Bovine perinatal mortality (death of the fetus or calf before, during, or within 48 h of calving at full term (≥260 days) may be caused by noninfectious and infectious causes. Although infectious causes of fetal mortality are diagnosed less frequently, infection in utero may also compromise the development of the fetus without causing death. This review presents fetomaternal responses to infection and the changes which can be observed in such cases. Response to infection, especially the concentration of immunoglobulins and some acute-phase proteins, may be used for diagnostic purposes. Some changes in internal organs may also be used as an indicator of infection in utero. However, in all cases (except pathogen-specific antibody response) non-pathogen-specific responses do not aid in pathogen-specific diagnosis of the cause of calf death. But, nonspecific markers of in utero infection may allow us to assign the cause of fetal mortality to infection and thus Citation: Jawor, P.; Mee, J.F.; increase our overall diagnosis rate, particularly in cases of the “unexplained stillbirth”. Stefaniak, T. Role of Infection and Immunity in Bovine Perinatal Abstract: Bovine perinatal mortality due to infection may result either from the direct effects of Mortality: Part 2. -

Mbio-2016-Casadevall-.Pdf
Downloaded from EDITORIAL Rigorous Science: a How-To Guide mbio.asm.org Arturo Casadevall,a Founding Editor in Chief, mBio, Ferric C. Fang,b Editor in Chief, Infection and Immunity Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USAa; Departments of Laboratory on January 3, 2017 - Published by Medicine and Microbiology, University of Washington School of Medicine, Seattle, Washington, USAb ABSTRACT Proposals to improve the reproducibility of biomedical research have emphasized scientific rigor. Although the word “rigor” is widely used, there has been little specific discussion as to what it means and how it can be achieved. We suggest that scientific rigor combines elements of mathematics, logic, philosophy, and ethics. We propose a framework for rigor that includes redundant experimental design, sound statistical analysis, recognition of error, avoidance of logical fallacies, and intel- lectual honesty. These elements lead to five actionable recommendations for research education. igor is a prized quality in scientific work. Although the term is ciple, such as Schrödinger’s cat or Maxwell’s demon in physics, or Rwidely used in both scientific and lay parlance, it has not been entirely experimental, as illustrated by Cavendish’s measurement precisely defined (1). Rigor has gained new prominence amid con- of the gravitational constant at the end of the 18th century. How- mbio.asm.org cerns about a lack of reproducibility in important studies (2, 3), an ever, in the biomedical sciences, most research has both theoreti- epidemic of retractions due to misconduct (4), and the discovery cal and experimental aspects. that the published literature is riddled with problematic images (5). -

The 100 Top-Cited Tuberculosis Research Studies
INT J TUBERC LUNG DIS 19(6):717–722 Q 2015 The Union http://dx.doi.org/10.5588/ijtld.14.0925 The 100 top-cited tuberculosis research studies L.-M. Chen,*† Y.-Q. Liu,* J.-N. Shen,* Y.-L. Peng,* T.-Y. Xiong,* X. Tong,‡ L. Du,*§¶ Y.-G. Zhang*§¶ *West China Hospital, West China Medical School, Sichuan University, Chengdu, †Department of Anaesthesiology, West China Hospital of Sichuan University, Chengdu, ‡Department of Respiratory Medicine, West China Hospital of Sichuan University, Chengdu, §The Periodical Press of West China Hospital, Sichuan University, Chengdu, ¶The Chinese Cochrane Centre, West China Hospital, Sichuan University, Chengdu, China SUMMARY The examination of top-cited studies is a useful method ing author. The United States contributed the largest for identify and monitoring outstanding scientific number of studies, followed by the United Kingdom and research. The objective of this study was to identify France. The institutions with the largest number of and analyse the characteristics of the top 100 cited articles were the Institut National de la Santeetdela´ research studies on tuberculosis (TB) based on the Web Recherche Medicale´ in France and the University of of Knowledge. Overall, the top 100 cited studies were California in the United States. The studies appeared in cited between 366 and 4443 times, and were published 35 journals, with 11 published in Science, followed by between 1995 and 2010, with the largest number of PNAS and NEJM. The majority of TB articles have been publications in 2003 and in 1995. Four studies were published in those medical journals with the highest attributed to a single author and 10 to two authors; the impact factors, and are from the most industrialised number of authors exceeded six in 50 studies. -

Elsevier Offers 950 New Health Titles to Research4life
October 12, 2011 Ylann Schemm Phone: +31 20 485 2025 E-mail:[email protected] Elsevier Offers 950 New Health Titles to Research4Life Leading health sciences publisher makes key Mosby, Saunders, Churchill Livingstone electronic titles freely available through UN developing world research access program Amsterdam, NL October 12, 2011 - Elsevier, a world-leading publisher of scientific, technical and medical information products and services, today announced that it is contributing an additional 950 electronic books to Research4Life, a public-private partnership working to achieve the UN’s Millennium Development Goals by providing developing world access to critical scientific research. Building on the 800 existing Elsevier science and technology books, the new electronic books cover Clinical Medicine (438 titles), Health Professions (332 titles), Veterinary Medicine (174 titles), and Clinical Dentistry (24 titles). These include seminal works such as Clinical Gynecology , Cancer Pain , Pain Medicine , Spinal Cord Injuries , and Saunders Manual of Small Animal Practice and will be accessible to users by the end of the year. "Health practitioners and researchers in developing countries will benefit greatly from this increased ebooks offering, said Kimberly Parker, HINARI Programme Manager at the WHO, “We greatly appreciate the efforts made by Research4Life partners to continuously improve and update their content offering to those who need it most.” “Contributing clinical titles helps to support doctors and nurses in the developing world -
Infection and Immunity Volume 51 * January 1986 * Number 1 J
INFECTION AND IMMUNITY VOLUME 51 * JANUARY 1986 * NUMBER 1 J. W. Shands, Jr., Editor in Chief (1989) University of Florida, Gainesville Phillip J. Baker, Editor (1990) Peter F. Bonventre, Editor (1989) Arthur G. Johnson, Editor (1986) National Institute ofAllergy and University of Cincinnati University of Minnesota, Duluth Infectious Diseases Cincinnati, Ohio Stephan E. Mergenhagen, Editor (1989) Bethesda, Md. Roy Curtiss III, Editor (1990) National Institute of Dental Researcl Edwin H. Beachey, Editor (1988) Washington University Bethesda, Md. VA Medical Center St. Louis, Mo. Memphis, Tenn. EDITORIAL BOARD Leonard C. Altman (1986) Toby K. Eisenstein (1987) F. Kierszenbaum (1987) Catherine Saelinger (1987) Michael A. Apicella (1988) Peter Elsbach (1986) Paul Kolenbrander (1986) Dwayne C. Savage (1987) Roland Arnold (1987) Stanley Falkow (1988) Julius P. Kreier (1986) Irving E. Salit (1986) John B. Bartlett (1988) John R. Finerty (1987) Maurice J. Lefford (1987) Charles F. Schachtele (1988) Joel B. Baseman (1988) Robert Fitzgerald (1986) Thomas Lehner (1986) Julius Schachter (1986) Robert E. Baughn (1987) James D. Folds (1988) Stephen H. Leppla (1988) Gary K. Schoolnik (1987) Gary K. Best (1988) Samuel B. Formal (1986) F. Y. Liew (1988) June R. Scott (1987) Jenefer Blackwell (1988) Peter Gemski (1988) Francis L. Macrina (1988) Philip Scott (1988) Arnold S. Bleiweis (1987) Robert Genco (1988) John Mansfield (1988) Alan Sher (1987) William H. Bowen (1988) Ronald J. Gibbons (1988) Jerry R. McGhee (1988) Gerald D. Shockman (1986) David E. Briles (1988) Emil Gotschlich (1988) Floyd C. McIntire (1988) W. A. Simpson (1988) Robert R. Brubaker (1986) Frank Griffin (1987) Monte Meltzer (1986) Phillip D. Smith (1988) Gerald Byrne (1988) Richard Guerrant (1986) Jiri Mestecky (1986) Ralph Snyderman (1988) Bruce Chassy (1987) Thomas L. -

AITKEN ALEXANDER London Book Fair 2019
AITKEN ALEXANDER ASSOCIATES London Book Fair 2019 For further information on all clients and titles in this catalogue, please contact: LISA BAKER France, Germany, Holland and Italy Email: [email protected] ANNA WATKINS Brazil, Bulgaria, China, Croatia, Czech Republic, Denmark, Estonia, Finland, Greece, Hungary, Iceland, Indonesia, Israel, Japan, Korea, Latvia, Lithuania, Macedonia, Norway, Portugal, Poland, Romania, Russia, Slovakia, Slovenia, Spain, Taiwan, Thailand and Turkey Email: [email protected] MONICA MACSWAN All Arabic and Indian language territories Email: [email protected] Literary Agents Centre Tables: Anna – 33f, Monica – 33e, Lisa – 34f For Film and Television Rights please contact: LESLEY THORNE Email: [email protected] Aitken Alexander Associates Ltd. 291 Gray’s Inn Road London WC1X 8QJ Telephone (020) 7373 8672 www.aitkenalexander.co.uk @AitkenAlexander @aitkenalexander Contents Page Fiction: The Wisdom of Bones by Kitty Aldridge p.1 Saltwater by Jessica Andrews p.2 The Body Lies by Jo Baker p.3 My Sister, the Serial Killer by Oyinkan Braithwaite p.4 In the Full Light of the Sun by Clare Clark p.5 Your Fault by Andrew Cowan p.6 This Brutal House by Niven Govinden p.7 The Porpoise by Mark Haddon p.8 Rabbit Foot Bill by Helen Humphreys p.9 The Harpy by Megan Hunter p.10 The Great Wide Open by Douglas Kennedy p.11 When We Were Rich by Tim Lott p.12 The Anthill by Julianne Pachico p.13 Lanny by Max Porter p.14 All the Water in the World by Karen Raney p.15 The Sandpit by Nicholas Shakespeare -

Access of E-Books During the COVID-19
Access of e-books during the COVID-19 Sl. No. ISBN Book Title Year Subject Imprint Shortcut URL 1 9780122384523 A Mathematical Introduction to Logic (Second Edition) 2001 Math/StatisticsAcademic Press https://www.sciencedirect.com/science/book/9780122384523 2 9780081001943 Aerodynamics for Engineering Students (Seventh Edition) 2015 Engineering Butterworth-Heinemannhttps://www.sciencedirect.com/science/book/9780081001943 3 9780080966328 Aerodynamics for Engineering Students (Sixth Edition) 2013 Engineering Butterworth-Heinemannhttps://www.sciencedirect.com/science/book/9780080966328 4 9780080969053 Aircraft Structures for Engineering Students (Fifth Edition) 2012 Engineering Butterworth-Heinemannhttps://www.sciencedirect.com/science/book/9780080969053 5 9780081009147 Aircraft Structures for Engineering Students (Sixth Edition) 2017 Engineering Butterworth-Heinemannhttps://www.sciencedirect.com/science/book/9780081009147 6 9780123848666 An Introduction to Dynamic Meteorology (Fifth Edition) 2013 Earth & EnvironmentalAcademic Science Press https://www.sciencedirect.com/science/book/9780123848666 7 9780120455911 An Introduction to Human Evolutionary Anatomy 1990 Life Sciences Academic Press https://www.sciencedirect.com/science/book/9780120455911 8 9780123742605 An Introduction to Parallel Programming 2011 Computing Morgan Kaufmann https://www.sciencedirect.com/science/book/9780123742605 9 9780123814166 An Introduction to Stochastic Modeling (Fourth Edition) 2011 Math/StatisticsAcademic Press https://www.sciencedirect.com/science/book/9780123814166 -

Form 20-F Annual Report 2006 on Form 20-F Annual Report 2006 on Form 20-F
169724 20-F Cover_v2.qxd 7/3/07 16:00 Page 1 > www.reedelsevier.com Reed Elsevier Form 20-F Annual Report 2006 on Form 20-F Annual Report 2006 on Form 20-F Annual Report 2006 on Form As filed with the Securities and Exchange Commission on March 22, 2007 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 20-F (Mark One) n REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) or 12(g) OF THE SECURITIES EXCHANGE ACT OF 1934 Or ¥ ANNUAL REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2006 Or n TRANSITION REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Or n SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 Commission file number: 1-3334 REED ELSEVIER PLC REED ELSEVIER NV (Exact name of Registrant as specified in its charter) (Exact name of Registrant as specified in its charter) England The Netherlands (Jurisdiction of incorporation or organisation) (Jurisdiction of incorporation or organisation) 1-3 Strand Radarweg 29 London WC2N 5JR 1043 NX Amsterdam England The Netherlands (Address of principal executive offices) (Address of principal executive offices) Securities registered or to be registered pursuant to Section 12(b) of the Act: Name of exchange on which Title of each class registered Reed Elsevier PLC: American Depositary Shares (each representing four Reed Elsevier PLC ordinary shares) New York Stock Exchange Ordinary shares of 12.5p each (the ""Reed Elsevier PLC ordinary shares'') New York Stock Exchange* Reed Elsevier NV: American Depositary Shares (each representing two Reed Elsevier NV ordinary shares) New York Stock Exchange Ordinary shares of 40.06 each (the ""Reed Elsevier NV ordinary shares'') New York Stock Exchange* * Listed, not for trading, but only in connection with the listing of the applicable Registrant's American Depositary Shares issued in respect thereof. -
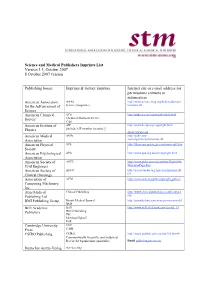
Science and Medical Publishers Imprints List Version 1.1, October 2007 8 October 2007 Version
Science and Medical Publishers Imprints List Version 1.1, October 2007 8 October 2007 version Publishing house Imprints & former imprints Internet site or e-mail address for permissions contacts or information American Association AAAS http://www.sciencemag.org/help/readers/per for the Advancement of Science (magazine) missions.dtl Science American Chemical ACS http://pubs.acs.org/copyright/index.html Society Chemical Abstracts Service CAS American Institute of AIP http://journals.aip.org/copyright.html Physics [include AIP member societies?] [email protected] American Medical AMA http://pubs.ama- Association assn.org/misc/permissions.dtl American Physical APS http://librarians.aps.org/permissionscopy.htm Society l American Psychological APA http://www.apa.org/about/copyright.html Association American Society of ASCE http://www.pubs.asce.org/authors/Rightslink Civil Engineers WelcomePage.htm American Society of ASCO http://jco.ascopubs.org/misc/permissions.sht Clinical Oncology ml Association of ACM http://www.acm.org/pubs/copyright_policy/ Computing Machinery, Inc. Atlas Medical Clinical Publishing http://www.clinicalpublishing.co.uk/contact. Publishing Ltd asp BMJ Publishing Group British Medical Journal http://journals.bmj.com/misc/permissions.dtl BMJ Brill Academic Brill http://www.brill.nl/default.aspx?partid=15 Publishers Hotei Publishing IDC Martinus Nijhoff VSP Cambridge University CUP Press CABI CSIRO Publishing CSIRO http://www.publish.csiro.au/nid/182.htm#8 Commonwealth Scientific and Industrial Research Organisation (Australia) -

Product ID Book Title Series Title Volume 9780123815040 Food Safety Management 9780123819888 Risk Management for Food Allergy 97
Product ID Book Title Series Title Volume 9780123815040 Food Safety Management 9780123819888 Risk Management for Food Allergy 9780123846884 Atlas of Drosophila Morphology 9780123858818 Handbook of Farm, Dairy and Food Machinery Engineering 9780123877376 Tomato Diseases 9780123914538 Mass Production of Beneficial Organisms 9780123919212 Food Industry Wastes 9780123945860 Genetics and the Behavior of Domestic Animals 9780123946010 Innovations in Food Packaging 9780123948014 The Agronomy and Economy of Turmeric and Ginger 9780123964908 Dictionary of Trees, Volume 2: South America 9780123964915 Sea Urchins DAFS/Developments in Aquaculture38 and Fisheries Science 9780123969538 Physiology of the Cladocera 9780123969552 Insect Resistance Management 9780123970039 Stock Identification Methods 9780123972040 Diagnosing Wild Species Harvest 9780123979353 Genetic and Genomic Resources of Grain Legume Improvement 9780123985293 Integrated Pest Management 9780123985309 Introduction to Food Engineering FST/Food Science and Technology 9780123985491 Flavour Science 9780124058781 Genetically Modified Food Sources 9780124158191 Physiological Systems in Insects 9780124158740 Insect Molecular Genetics 9780124159235 Food Process Engineering and Technology FST/Food Science and Technology 9780124160286 Sexual Selection 9780124160415 Foodborne Infections and Intoxications FST/Food Science and Technology 9780124166479 Catalogue of the Cicadoidea (Hemiptera: Auchenorrhyncha) 9780124171954 Transparency for Sustainability in the Food Chain 9780857090379 Diet Immunity