Mayo Clinic Proceedings: Innovations, Quality & Outcomes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BOOKLIST for ADN Program Spring Quarter (January 4, 2021)
BOOKLIST for ADN Program Spring Quarter (January 4, 2021) The following outlines the resources (textbooks, websites, media, etc.) utilized in each course. Required resources are necessary for academic success: you must obtain and study from them to succeed in a course as outlined in the course syllabus. Recommended resources provide material that will boost your academic performance in that course but are not always necessary for academic success. Faculty may or may not use recommended resources in class or during clinical. Please confer with course faculty prior to purchasing recommended resources. Use International Standard Book Numbers (ISBN) to identify the books below with any book vendor. Rented, used, and electronic formats of the resources may be available at a reduced cost to the student. A dagger (†) preceding a title indicates the resource is appropriate for renting. A keyboard symbol (⌨) preceding a title indicates the resource is available electronically/online. When renting a textbook, it may not include CDs, DVDs, or access codes for other protected electronic content, even if the book had those features when sold new. These features may be required for successful completion of the course in which the book is used. If you believe a textbook is supposed to include a disk or an unused access code, check before renting. Galen is not responsible for purchases made in error. Textbooks/resources may be used in multiple courses throughout the program. Students should verify this information via the booklist prior to purchasing/renting or returning/reselling. Galen encourages students to retain textbooks/resources as reference materials to assist throughout their nursing career. -

Elsevier Offers 950 New Health Titles to Research4life
October 12, 2011 Ylann Schemm Phone: +31 20 485 2025 E-mail:[email protected] Elsevier Offers 950 New Health Titles to Research4Life Leading health sciences publisher makes key Mosby, Saunders, Churchill Livingstone electronic titles freely available through UN developing world research access program Amsterdam, NL October 12, 2011 - Elsevier, a world-leading publisher of scientific, technical and medical information products and services, today announced that it is contributing an additional 950 electronic books to Research4Life, a public-private partnership working to achieve the UN’s Millennium Development Goals by providing developing world access to critical scientific research. Building on the 800 existing Elsevier science and technology books, the new electronic books cover Clinical Medicine (438 titles), Health Professions (332 titles), Veterinary Medicine (174 titles), and Clinical Dentistry (24 titles). These include seminal works such as Clinical Gynecology , Cancer Pain , Pain Medicine , Spinal Cord Injuries , and Saunders Manual of Small Animal Practice and will be accessible to users by the end of the year. "Health practitioners and researchers in developing countries will benefit greatly from this increased ebooks offering, said Kimberly Parker, HINARI Programme Manager at the WHO, “We greatly appreciate the efforts made by Research4Life partners to continuously improve and update their content offering to those who need it most.” “Contributing clinical titles helps to support doctors and nurses in the developing world -

AITKEN ALEXANDER London Book Fair 2019
AITKEN ALEXANDER ASSOCIATES London Book Fair 2019 For further information on all clients and titles in this catalogue, please contact: LISA BAKER France, Germany, Holland and Italy Email: [email protected] ANNA WATKINS Brazil, Bulgaria, China, Croatia, Czech Republic, Denmark, Estonia, Finland, Greece, Hungary, Iceland, Indonesia, Israel, Japan, Korea, Latvia, Lithuania, Macedonia, Norway, Portugal, Poland, Romania, Russia, Slovakia, Slovenia, Spain, Taiwan, Thailand and Turkey Email: [email protected] MONICA MACSWAN All Arabic and Indian language territories Email: [email protected] Literary Agents Centre Tables: Anna – 33f, Monica – 33e, Lisa – 34f For Film and Television Rights please contact: LESLEY THORNE Email: [email protected] Aitken Alexander Associates Ltd. 291 Gray’s Inn Road London WC1X 8QJ Telephone (020) 7373 8672 www.aitkenalexander.co.uk @AitkenAlexander @aitkenalexander Contents Page Fiction: The Wisdom of Bones by Kitty Aldridge p.1 Saltwater by Jessica Andrews p.2 The Body Lies by Jo Baker p.3 My Sister, the Serial Killer by Oyinkan Braithwaite p.4 In the Full Light of the Sun by Clare Clark p.5 Your Fault by Andrew Cowan p.6 This Brutal House by Niven Govinden p.7 The Porpoise by Mark Haddon p.8 Rabbit Foot Bill by Helen Humphreys p.9 The Harpy by Megan Hunter p.10 The Great Wide Open by Douglas Kennedy p.11 When We Were Rich by Tim Lott p.12 The Anthill by Julianne Pachico p.13 Lanny by Max Porter p.14 All the Water in the World by Karen Raney p.15 The Sandpit by Nicholas Shakespeare -

Access of E-Books During the COVID-19
Access of e-books during the COVID-19 Sl. No. ISBN Book Title Year Subject Imprint Shortcut URL 1 9780122384523 A Mathematical Introduction to Logic (Second Edition) 2001 Math/StatisticsAcademic Press https://www.sciencedirect.com/science/book/9780122384523 2 9780081001943 Aerodynamics for Engineering Students (Seventh Edition) 2015 Engineering Butterworth-Heinemannhttps://www.sciencedirect.com/science/book/9780081001943 3 9780080966328 Aerodynamics for Engineering Students (Sixth Edition) 2013 Engineering Butterworth-Heinemannhttps://www.sciencedirect.com/science/book/9780080966328 4 9780080969053 Aircraft Structures for Engineering Students (Fifth Edition) 2012 Engineering Butterworth-Heinemannhttps://www.sciencedirect.com/science/book/9780080969053 5 9780081009147 Aircraft Structures for Engineering Students (Sixth Edition) 2017 Engineering Butterworth-Heinemannhttps://www.sciencedirect.com/science/book/9780081009147 6 9780123848666 An Introduction to Dynamic Meteorology (Fifth Edition) 2013 Earth & EnvironmentalAcademic Science Press https://www.sciencedirect.com/science/book/9780123848666 7 9780120455911 An Introduction to Human Evolutionary Anatomy 1990 Life Sciences Academic Press https://www.sciencedirect.com/science/book/9780120455911 8 9780123742605 An Introduction to Parallel Programming 2011 Computing Morgan Kaufmann https://www.sciencedirect.com/science/book/9780123742605 9 9780123814166 An Introduction to Stochastic Modeling (Fourth Edition) 2011 Math/StatisticsAcademic Press https://www.sciencedirect.com/science/book/9780123814166 -

Form 20-F Annual Report 2006 on Form 20-F Annual Report 2006 on Form 20-F
169724 20-F Cover_v2.qxd 7/3/07 16:00 Page 1 > www.reedelsevier.com Reed Elsevier Form 20-F Annual Report 2006 on Form 20-F Annual Report 2006 on Form 20-F Annual Report 2006 on Form As filed with the Securities and Exchange Commission on March 22, 2007 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 20-F (Mark One) n REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) or 12(g) OF THE SECURITIES EXCHANGE ACT OF 1934 Or ¥ ANNUAL REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2006 Or n TRANSITION REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Or n SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 Commission file number: 1-3334 REED ELSEVIER PLC REED ELSEVIER NV (Exact name of Registrant as specified in its charter) (Exact name of Registrant as specified in its charter) England The Netherlands (Jurisdiction of incorporation or organisation) (Jurisdiction of incorporation or organisation) 1-3 Strand Radarweg 29 London WC2N 5JR 1043 NX Amsterdam England The Netherlands (Address of principal executive offices) (Address of principal executive offices) Securities registered or to be registered pursuant to Section 12(b) of the Act: Name of exchange on which Title of each class registered Reed Elsevier PLC: American Depositary Shares (each representing four Reed Elsevier PLC ordinary shares) New York Stock Exchange Ordinary shares of 12.5p each (the ""Reed Elsevier PLC ordinary shares'') New York Stock Exchange* Reed Elsevier NV: American Depositary Shares (each representing two Reed Elsevier NV ordinary shares) New York Stock Exchange Ordinary shares of 40.06 each (the ""Reed Elsevier NV ordinary shares'') New York Stock Exchange* * Listed, not for trading, but only in connection with the listing of the applicable Registrant's American Depositary Shares issued in respect thereof. -
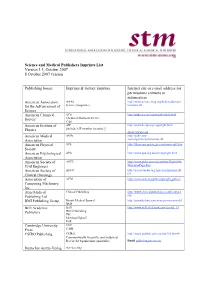
Science and Medical Publishers Imprints List Version 1.1, October 2007 8 October 2007 Version
Science and Medical Publishers Imprints List Version 1.1, October 2007 8 October 2007 version Publishing house Imprints & former imprints Internet site or e-mail address for permissions contacts or information American Association AAAS http://www.sciencemag.org/help/readers/per for the Advancement of Science (magazine) missions.dtl Science American Chemical ACS http://pubs.acs.org/copyright/index.html Society Chemical Abstracts Service CAS American Institute of AIP http://journals.aip.org/copyright.html Physics [include AIP member societies?] [email protected] American Medical AMA http://pubs.ama- Association assn.org/misc/permissions.dtl American Physical APS http://librarians.aps.org/permissionscopy.htm Society l American Psychological APA http://www.apa.org/about/copyright.html Association American Society of ASCE http://www.pubs.asce.org/authors/Rightslink Civil Engineers WelcomePage.htm American Society of ASCO http://jco.ascopubs.org/misc/permissions.sht Clinical Oncology ml Association of ACM http://www.acm.org/pubs/copyright_policy/ Computing Machinery, Inc. Atlas Medical Clinical Publishing http://www.clinicalpublishing.co.uk/contact. Publishing Ltd asp BMJ Publishing Group British Medical Journal http://journals.bmj.com/misc/permissions.dtl BMJ Brill Academic Brill http://www.brill.nl/default.aspx?partid=15 Publishers Hotei Publishing IDC Martinus Nijhoff VSP Cambridge University CUP Press CABI CSIRO Publishing CSIRO http://www.publish.csiro.au/nid/182.htm#8 Commonwealth Scientific and Industrial Research Organisation (Australia) -

Product ID Book Title Series Title Volume 9780123815040 Food Safety Management 9780123819888 Risk Management for Food Allergy 97
Product ID Book Title Series Title Volume 9780123815040 Food Safety Management 9780123819888 Risk Management for Food Allergy 9780123846884 Atlas of Drosophila Morphology 9780123858818 Handbook of Farm, Dairy and Food Machinery Engineering 9780123877376 Tomato Diseases 9780123914538 Mass Production of Beneficial Organisms 9780123919212 Food Industry Wastes 9780123945860 Genetics and the Behavior of Domestic Animals 9780123946010 Innovations in Food Packaging 9780123948014 The Agronomy and Economy of Turmeric and Ginger 9780123964908 Dictionary of Trees, Volume 2: South America 9780123964915 Sea Urchins DAFS/Developments in Aquaculture38 and Fisheries Science 9780123969538 Physiology of the Cladocera 9780123969552 Insect Resistance Management 9780123970039 Stock Identification Methods 9780123972040 Diagnosing Wild Species Harvest 9780123979353 Genetic and Genomic Resources of Grain Legume Improvement 9780123985293 Integrated Pest Management 9780123985309 Introduction to Food Engineering FST/Food Science and Technology 9780123985491 Flavour Science 9780124058781 Genetically Modified Food Sources 9780124158191 Physiological Systems in Insects 9780124158740 Insect Molecular Genetics 9780124159235 Food Process Engineering and Technology FST/Food Science and Technology 9780124160286 Sexual Selection 9780124160415 Foodborne Infections and Intoxications FST/Food Science and Technology 9780124166479 Catalogue of the Cicadoidea (Hemiptera: Auchenorrhyncha) 9780124171954 Transparency for Sustainability in the Food Chain 9780857090379 Diet Immunity -

Access to Business Research Resources Through Academic Library Web Sites: a Survey John C
Western Kentucky University TopSCHOLAR® DLPS Faculty Publications Library Public Services 2011 Access to Business Research Resources through Academic Library Web Sites: A Survey John C. Gottfried Western Kentucky University, [email protected] Follow this and additional works at: http://digitalcommons.wku.edu/dlps_fac_pub Part of the Curriculum and Instruction Commons, Library and Information Science Commons, Marketing Commons, Other Education Commons, Other Social and Behavioral Sciences Commons, and the Technology and Innovation Commons Recommended Repository Citation Gottfried, John C.. (2011). Access to Business Research Resources through Academic Library Web Sites: A Survey. Journal of Business & Finance Librarianship, 16 (1), 1-32. Original Publication URL: DOI: 10.1080/08963568.2011.530852 Available at: http://digitalcommons.wku.edu/dlps_fac_pub/34 This Article is brought to you for free and open access by TopSCHOLAR®. It has been accepted for inclusion in DLPS Faculty Publications by an authorized administrator of TopSCHOLAR®. For more information, please contact [email protected]. Business Resources 1 Running head: BUSINESS RESOURCES THROUGH ACADEMIC WEB SITES Access to Business Research Resources through Academic Library Web Sites: A Survey John C. Gottfried Western Kentucky University Contact: John Gottfried Business Librarian Western Kentucky University Libraries 1906 College Heights Blvd. #11067 Bowling Green, KY 42101-1067 , Email: [email protected] Voice: (270) 745-6176 Final version published in The Journal of Business & Finance Librarianship (2011), 16: 1, 1 — 32. Business Resources 2 Abstract This study is an examination of access to business research resources through academic library Web sites, including research databases, catalog services, research guides, and business librarians. The Web sites of 114 academic libraries serving top business programs in the United States were studied. -

The Efficient Team-Driven Quality Scholarship Model
Collaborative Librarianship Volume 12 Issue 1 Article 10 6-7-2020 The Efficienteam-Driv T en Quality Scholarship Model: A Process Evaluation of Collaborative Research Johanna Alexander California State University, Bakersfield, [email protected] Andrea Anderson California State University, Bakersfield, [email protected] Sandra Bozarth California State University, Bakersfield, [email protected] Heather Cribbs California State University, Bakersfield, [email protected] Kristine Holloway California State University, Bakersfield, [email protected] See next page for additional authors Follow this and additional works at: https://digitalcommons.du.edu/collaborativelibrarianship Part of the Scholarly Publishing Commons Recommended Citation Alexander, Johanna; Anderson, Andrea; Bozarth, Sandra; Cribbs, Heather; Holloway, Kristine; Livingston, Christopher; Overduin, Terezita; and Zhong, Ying (2020) "The Efficienteam-Driv T en Quality Scholarship Model: A Process Evaluation of Collaborative Research," Collaborative Librarianship: Vol. 12 : Iss. 1 , Article 10. Available at: https://digitalcommons.du.edu/collaborativelibrarianship/vol12/iss1/10 This Peer Reviewed Article is brought to you for free and open access by Digital Commons @ DU. It has been accepted for inclusion in Collaborative Librarianship by an authorized editor of Digital Commons @ DU. For more information, please contact [email protected],[email protected]. The Efficienteam-Driv T en Quality Scholarship Model: A Process Evaluation of Collaborative Research Cover Page Footnote -

Potent Marijuana Edibles Can Pose a Major Unrecognized Risk to Patients with Cardiovascular Disease
NEWS RELEASE UNDER EMBARGO UNTIL FEBRUARY 11, 2019, 12:01 AM ET Media contact: Eileen Leahy Elsevier +1 732 238 3628 [email protected] Potent marijuana edibles can pose a major unrecognized risk to patients with cardiovascular disease With widespread legalization and increasing use, more care, education, and research are needed about how each formulation of marijuana may affect and sometimes compromise the cardiovascular system of our aging population, according to the Canadian Journal of Cardiology Philadelphia, February 11, 2019 – As marijuana legalization sweeps North America, use of the substance has been on the rise, and the public’s attitude is shifting. An increasing number of people believe that “weed” is the safest recreational drug, one that carries health benefits that outweigh its risks. Those assumptions are challenged in an article and editorial published in the Canadian Journal of Cardiology that examine the story of a patient who developed crushing chest pain and myocardial ischemia after consuming most of a marijuana lollipop. “Marijuana can be a useful tool for many patients, especially for pain and nausea relief. At the same time, like all other medications, it does carry risk and side effects. In a recent case, inappropriate dosing and oral consumption of marijuana by an older patient with stable cardiovascular disease resulted in distress that caused a cardiac event and subsequent reduced cardiac function,” said Alexandra Saunders, MD, Dalhousie University, Internal Medicine Program and Horizon Health Network’s Department of Cardiology, Saint John, NB, Canada. The case report describes a 70-year-old man with stable coronary artery disease, taking the appropriate cardiac medications, who ate most of a lollipop that was infused with 90 mg of THC (delta-9- tetrahydrocannabinol) to relieve pain and aid sleep, which caused him to have a potentially-serious heart attack. -

W.B. Saunders Company an Imprint of Elsevier Inc. 11830 Westline Industrial Drive St. Louis, Missouri 63146 EQUINE GERIATRIC
W.B. Saunders Company An Imprint of Elsevier Inc. 11830 Westline Industrial Drive St. Louis, Missouri 63146 EQUINE GERIATRIC MEDICINE AND SURGERY ISBN-10: 0-7216-0163-4 © 2006 Elsevier Inc.All rights reserved. ISBN-13: 978-0-7216-0163-2 Back cover photos © 2006 Katie Barrett No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechani- cal, including photocopy,recording, or any information storage and retrieval system, without permission in writing from the publisher. Permissions may be sought directly from Elsevier Inc. Rights Department in Philadelphia, USA: phone: (+1)215 238 7869, fax: (+1) 215238 2239, email: [email protected] may also complete your request on- line via the Elsevier Science homepage (http://www.elsevier.com, by selecting “Customer Support” and then “Obtaining Permissions.” Notice Veterinary Medicine is an ever-changing field.Standard safety precautions must be followed but as new research and clinical experience broaden our knowledge,changes in treatment and drug therapy may become necessary or appropriate.Readers are advised to check the most current product information provided by the manufacturer of each drug to be administered to verify the recommended dose,the method and duration of administration,and contraindications.It is the responsibility of the treating veterinarian,relying on experience and knowledge of the patient,to determine dosages and the best treatment for each individual patient.Neither the Publisher nor the author assumes any liability -

A Short History of Elsevier
A SHORT HISTORY OF ELSEVIER In Celebration of the 125th Anniversary of Elsevier and the 425th Anniversary of the House of Elzevir Scientists and healthcare professionals are more inclined to look forward than back, which perhaps explains why there are so few written accounts of Elsevier, in spite of the publishing company’s long and successful history. hereas historians have recorded science Press, to name but a few of the companies that Wand medicine’s key moments of are now part of the Elsevier family, bringing with progress — from Galileo’s celestial revelations them long and rich histories of their own. As the to Fleming’s discovery of penicillin to the recent company moves forward into the new millennium, identification of SARS as a Corona virus — few its founding motto seems more apt than ever: have taken the time to examine the role that Non Solus (not alone). publishers have played in the history of science. Given that 2005 marks the 125th birthday Founding Principles of Elsevier and the 425th anniversary of the publishing house of Elzevir from which the t is ironic that Elsevier’s founder, Jacobus modern company takes its name, the time seems IRobbers, chose to name his small Dutch right to redress that imbalance and reflect on publishing company after a defunct seventeenth the myriad ways in which Elsevier has played a century publishing house. Nevertheless it makes role in the history of science over the last 125 sense: for in spite of the fact that the House of years. In that time Elsevier has evolved from a Elzevir had been out of business since 1712, the small Dutch publishing house devoted to the promulgation of classical reputation of Elzevir publications had grown rather than declined by scholarship to an international multimedia publishing company that March of 1880 when the modern Elsevier was founded.