The Metabolic Role of the Hippo Pathway in Liver Development and Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacological Targeting of the Mitochondrial Phosphatase PTPMT1 by Dahlia Doughty Shenton Department of Biochemistry Duke
Pharmacological Targeting of the Mitochondrial Phosphatase PTPMT1 by Dahlia Doughty Shenton Department of Biochemistry Duke University Date: May 1 st 2009 Approved: ___________________________ Dr. Patrick J. Casey, Supervisor ___________________________ Dr. Perry J. Blackshear ___________________________ Dr. Anthony R. Means ___________________________ Dr. Christopher B. Newgard ___________________________ Dr. John D. York Dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Biochemistry in the Graduate School of Duke University 2009 ABSTRACT Pharmacological Targeting of the Mitochondrial Phosphatase PTPMT1 by Dahlia Doughty Shenton Department of Biochemistry Duke University Date: May 1 st 2009 Approved: ___________________________ Dr. Patrick J. Casey, Supervisor ___________________________ Dr. Perry J. Blackshear ___________________________ Dr. Anthony R. Means ___________________________ Dr. Christopher B. Newgard ___________________________ Dr. John D. York An abstract of a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Biochemistry in the Graduate School of Duke University 2009 Copyright by Dahlia Doughty Shenton 2009 Abstract The dual specificity protein tyrosine phosphatases comprise the largest and most diverse group of protein tyrosine phosphatases and play integral roles in the regulation of cell signaling events. The dual specificity protein tyrosine phosphatases impact multiple -

Structural and Functional Analysis of PTPMT1, a Phosphatase Required for Cardiolipin Synthesis
Structural and functional analysis of PTPMT1, a phosphatase required for cardiolipin synthesis Junyu Xiaoa,1, James L. Engela,1, Ji Zhanga, Mark J. Chenb, Gerard Manningb, and Jack E. Dixona,c,d,e,2 aDepartment of Pharmacology, University of California, La Jolla, CA 92093; bRazavi Newman Center for Bioinformatics, Salk Institute, La Jolla, CA 92037; cDepartment of Cellular and Molecular Medicine, University of California, La Jolla, CA 92093; dDepartment of Chemistry and Biochemistry, University of California, La Jolla, CA 92093; and eHoward Hughes Medical Institute, Chevy Chase, MD 20815 Contributed by Jack E. Dixon, June 9, 2011 (sent for review December 29, 2010) PTPMT1 (PTP localized to the Mitochondrion 1) is a member of the chondria, and its level is not affected by the loss of PTPMT1, sug- protein tyrosine phosphatase superfamily that is localized exclu- gesting that PI(5)P is not its endogenous substrate (10). We sively to the mitochondrion. We recently reported that PTPMT1 recently demonstrated that the physiological target of PTPMT1 dephosphorylates phosphatidylglycerol phosphate, an essential is phosphatidylglycerol phosphate (PGP) (11), which is structu- intermediate of cardiolipin biosynthesis. To gain further insights rally remarkably similar to PI(5)P (Fig. 1C). into the molecular basis of PTPMT1 function, we determined the Phosphatidylglycerol (PG), the product of PTPMT1’s activity, crystal structures of the phosphatase domain of PTPMT1. PTPMT1 is an essential component of pulmonary surfactant, and a precur- exhibits a canonical protein tyrosine phosphatase domain fold, sor for cardiolipin biosynthesis (Fig. 1D and Fig. S2). Cardiolipin resembling many dual-specificity phosphatases such as phospha- is a glycerophospholipid found predominantly in the mitochon- tase and tensin homolog and vaccinia H1-related phosphatase. -

Revealing the Action Mechanisms of Dexamethasone on the Birth Weight
® Observational Study Medicine OPEN Revealing the action mechanisms of dexamethasone on the birth weight of infant using RNA-sequencing data of trophoblast cells ∗ Hongkai Shang, MDa, Liping Sun, BMa, Thorsten Braun, MDb, Qi Si, BMa, Jinyi Tong, MMa, Abstract Dexamethasone (DEX) could induce low birth weight of infant, and low birth weight has close associations with glucocorticoid levels, insulin resistance, hypertension, and metabolic syndrome in adulthood. This study was designed to reveal the action mechanisms of DEX on the birth weight of infant. Using quantitative real-time polymerase chain reaction (qRT-PCR), trophoblast cells of human placenta were identified and the optimum treatment time of DEX were determined. Trophoblast cells were treated by DEX (DEX group) or ethanol (control group) (each group had 3 samples), and then were performed with RNA-sequencing. Afterward, the differentially expressed genes (DEGs) were identified by R package, and their potential functions were successively enriched using DAVID database and Enrichr method. Followed by protein–protein interaction (PPI) network was constructed using Cytoscape software. Using Enrichr method and TargetScan software, the transcription factors (TFs) and micorRNAs (miRNAs) targeted the DEGs separately were predicted. Based on MsigDB database, gene set enrichment analysis (GSEA) was performed. There were 391 DEGs screened from the DEX group. Upregulated SRR and potassium voltage-gated channel subfamily J member 4(KCNJ4) and downregulated GALNT1 separately were enriched in PDZ (an acronym of PSD-95, Dlg, and ZO-1) domain binding and Mucin type O-glycan biosynthesis. In the PPI network, CDK2 and CDK4 had higher degrees. TFs ATF2 and E2F4 and miRNA miR-16 were predicted for the DEGs. -
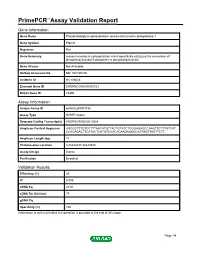
Primepcr™Assay Validation Report
PrimePCR™Assay Validation Report Gene Information Gene Name Phosphatidylglycerophosphatase and protein-tyrosine phosphatase 1 Gene Symbol Ptpmt1 Organism Rat Gene Summary mouse homolog is a phosphatase which specifically catalyzes the conversion of phosphatidylinositol 5-phosphate to phosphatidylinositol Gene Aliases Not Available RefSeq Accession No. NM_001105726 UniGene ID Rn.108023 Ensembl Gene ID ENSRNOG00000009723 Entrez Gene ID 29390 Assay Information Unique Assay ID qRnoCED0003185 Assay Type SYBR® Green Detected Coding Transcript(s) ENSRNOT00000013584 Amplicon Context Sequence AGCGCTTTGTCCTTTAACATGTTACTGTATCTCGGAGAGCCAAGTTCTTTGTCAT CCACAGACTTCATACTCATGTCAGCACAAGAGACCAGTAGTTAGTTCTT Amplicon Length (bp) 74 Chromosome Location 3:86444437-86444540 Assay Design Exonic Purification Desalted Validation Results Efficiency (%) 99 R2 0.999 cDNA Cq 20.91 cDNA Tm (Celsius) 79 gDNA Cq Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 1/4 PrimePCR™Assay Validation Report Ptpmt1, Rat Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak Melt curve analysis of above amplification Standard Curve Standard curve generated using 20 million copies of template diluted 10-fold to 20 copies Page 2/4 PrimePCR™Assay Validation Report Products used to generate validation data Real-Time PCR Instrument CFX384 Real-Time PCR Detection System Reverse Transcription Reagent iScript™ Advanced cDNA Synthesis Kit for RT-qPCR Real-Time PCR Supermix SsoAdvanced™ SYBR® Green Supermix Experimental Sample qPCR Reference Total RNA Data Interpretation Unique Assay ID This is a unique identifier that can be used to identify the assay in the literature and online. Detected Coding Transcript(s) This is a list of the Ensembl transcript ID(s) that this assay will detect. -

Identification of Potential Core Genes in Sevoflurance Induced Myocardial
Identication of Potential core genes in Sevourance induced Myocardial Energy Metabolism in Patients Undergoing Off-pump Coronary Artery Bypass Graft Surgery using Bioinformatics analysis Hua Lin ( [email protected] ) Tianjin Medical University General Hospital Airport Site Research article Keywords: sevourane, Myocardial Energy Metabolism, Off-pump Coronary Artery Bypass Graft Surgery Posted Date: November 18th, 2019 DOI: https://doi.org/10.21203/rs.2.17434/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/15 Abstract Background: Myocardial ischemia-reperfusion injury always happened after Off-pump coronary artery bypass graft(OPCABG), and this can not be avoided altogether. In this study, we tried to detect potential genes of sevourane-induced myocardial energy metabolism in patients undergoing OPCABG using bioinformatics analysis. Methods: We download and analyze the gene expression prole data from the Gene Expression Omnibus(GEO) database using bioinformatics methods. We downloded the gene expression data from the Gene Expression Omnibus(GEO) database using bioinformatics methods. Gene Ontology(GO) functional annotation analysis and Kyoto Encyclopedia of Genes and Genomes(KEGG) pathway enrichment analysis were used to analysis the screened differentially expressed genes(DEGs). Then, we established a protein–protein interaction (PPI) network to nd hub genes associated with myocardial energy metabolism. Results: Through PPI network, we nd ten hub genes, including JUN, EGR1, ATF3, FOSB, JUNB, DUSP1, EGR2, NR4A1, BTG2, NR4A2. Conclusions: In conclusion, the proteins encoded by EGR1ATF3c-FosBtg2JunBDUSP1NR4A1BTG2 and NR4A2 were related to cardiac function. ATF3, FOSB, JUNB, DUSP1, NR4A1, NR4A2 are related to apoptosis of cardiomyocytes. The protein encoded by BTG2 is related to hypertrophy. -

Host Protein Interactions
scientificscientificreport report Structure homology and interaction redundancy for discovering virus–host protein interactions Benoıˆt de Chassey 1,Laure`ne Meyniel-Schicklin 2,3, Anne Aublin-Gex 2,3, Vincent Navratil 2,3,w, Thibaut Chantier 2,3, Patrice Andre´1,2,3 &VincentLotteau2,3+ 1Hospices Civils de Lyon, Hoˆpital de la Croix-Rousse, Laboratoire de virologie, Lyon , 2Inserm, U1111, Lyon , and 3Universite´ de Lyon, Lyon, France Virus–host interactomes are instrumental to understand global purification coupled to mass spectrometry, led to the publication perturbations of cellular functions induced by infection and of the first virus–host interactomes [3–4]. Although incorporation discover new therapies. The construction of such interactomes is, of data from different methods or variation of the same method [5] however, technically challenging and time consuming. Here we has improved the quality of the data sets, diversification of describe an original method for the prediction of high-confidence methods is still clearly needed to generate high-quality interactions between viral and human proteins through comprehensive virus–host interactomes. In addition, regarding a combination of structure and high-quality interactome data. the size of host genome and the huge diversity of viruses, millions Validation was performed for the NS1 protein of the influenza of binary interactions remain to be tested. Therefore, accurate and virus, which led to the identification of new host factors that rapid methods for the identification of cellular interactors control viral replication. controlling viral replication is a major issue and a crucial step Keywords: interactome; prediction; protein interaction; towards the selection of original therapeutic targets and drug structure; virus development. -

Nerodia Fasciata/Clarkii</Em>
Clemson University TigerPrints All Theses Theses December 2019 Testing the Waters: Diversification and Selection in the Nerodia fasciata/clarkii Species Complex Mark Alexander DiMeo Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_theses Recommended Citation DiMeo, Mark Alexander, "Testing the Waters: Diversification and Selection in the Nerodia fasciata/clarkii Species Complex" (2019). All Theses. 3202. https://tigerprints.clemson.edu/all_theses/3202 This Thesis is brought to you for free and open access by the Theses at TigerPrints. It has been accepted for inclusion in All Theses by an authorized administrator of TigerPrints. For more information, please contact [email protected]. TESTING THE WATERS: DIVERSIFICATION AND SELECTION IN THE NERODIA FASCIATA/CLARKII SPECIES COMPLEX A Thesis Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Master of Science Biological Sciences by Mark Alexander DiMeo December 2019 Accepted by: Dr. Christopher Parkinson, Committee Chair Dr. J. Antonio Baeza Dr. Vincent Richards ABSTRACT Understanding evolution is a key component in trying to decipher the processes generating global species diversity. The strength, direction, and interaction of gene flow and selection often determine diversification patterns and the process of speciation. The Nerodia fasciata/clarkii species complex, a lineage of water snakes, is thought to have high levels of both gene flow and selective pressures due to ecological constraint. Nerodia clarkii resides in salt marsh and estuarine habitats while Nerodia fasciata is typically found in fresh water. Salinity is a strong selective pressure and is thought to play a role in the diversification process. -

Molecular Expression Analyses of Mice Treated with Antipsychotic Drugs
Molecular Expression Analyses of Mice Treated with Antipsychotic Drugs Carlotta Elizabeth Duncan A thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy The University of New South Wales January 2008 Supervisors: Professor Peter R. Schofield & Professor Cynthia Shannon Weickert ABSTRACT Schizophrenia is a devastating psychiatric disorder that affects approximately 1% of the population. The main treatments for schizophrenia are antipsychotic drugs that target dopamine receptors, yet the underlying biological mechanisms through which they alleviate the symptoms of schizophrenia remain ill defined. In this study, we used microarray analysis to profile the expression changes of thousands of genes simultaneously, following antipsychotic drug treatment of mice. Mice were treated chronically (28 days), or for a novel intermediate time-point (7 days), with one of three antipsychotic drugs: clozapine, haloperidol or olanzapine. The use of three drugs enabled us to discern antipsychotic-specific effects co-regulated by multiple drugs, rather than the side effects of individual compounds. Transcript profiling and validation by quantitative PCR of whole brain tissue revealed antipsychotic drug regulation of genes in diverse biological pathways, including: dopamine metabolism, neuropeptide and second-messenger signalling, neurogenesis, synaptic plasticity, cell adhesion, myelination, and voltage-gated ion channels. The regulation of voltage-gated channels by antipsychotic drugs has been suggested previously by electrophysiological studies, although thorough analysis has not been undertaken in vivo. Therefore, the second aim of this study was to characterise the regional mRNA and protein expression of two genes altered by multiple APDs, the voltage-gated potassium channel -subunit (Kcna1) and voltage- gated potassium channel interacting protein (Kchip3). -

Interindividual Variation in Gene Expression Responses and Metabolite Formation in Acetaminophen‑Exposed Primary Human Hepatocytes
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Arch Toxicol (2016) 90:1103–1115 DOI 10.1007/s00204-015-1545-2 TOXICOGENOMICS Interindividual variation in gene expression responses and metabolite formation in acetaminophen-exposed primary human hepatocytes Marlon J. A. Jetten1 · Ainhoa Ruiz-Aracama2 · Maarten L. J. Coonen1 · Sandra M. Claessen1 · Marcel H. M. van Herwijnen1 · Arjen Lommen2 · Joost H. M. van Delft1 · Ad A. C. M. Peijnenburg2 · Jos C. S. Kleinjans1 Received: 6 January 2015 / Accepted: 3 June 2015 / Published online: 24 June 2015 © The Author(s) 2015. This article is published with open access at Springerlink.com Abstract Acetaminophen (APAP) is a readily available high interindividual variation in expression were involved over-the-counter drug and is one of the most commonly include liver regeneration, inflammatory responses, mito- used analgesics/antipyretics worldwide. Large interindi- chondrial stress responses, hepatocarcinogenesis, cell cycle, vidual variation in susceptibility toward APAP-induced and drug efficacy. Additionally, the interindividual variation liver failure has been reported. However, the exact under- in the expression of these genes could be associated with the lying factors causing this variability in susceptibility are variability in expression levels of hydroxyl/methoxy-APAP still largely unknown. The aim of this study was to better and C8H13O5N-APAP-glucuronide. The before-mentioned understand this variability in response to APAP by evaluat- metabolites or their derivatives have also been reported in ing interindividual differences in gene expression changes blood of humans exposed to therapeutic APAP doses. Possi- and APAP metabolite formation in primary human hepato- bly these findings can contribute to elucidating the causative cytes (PHH) from several donors (n 5) exposed in vitro factors of interindividual susceptibility toward APAP. -

Zea Mays L.) Inbred Line Y423
International Journal of Molecular Sciences Article iTRAQ-Based Quantitative Proteomic Analysis of Embryogenic and Non-embryogenic Calli Derived from a Maize (Zea mays L.) Inbred Line Y423 Beibei Liu †, Xiaohui Shan †, Ying Wu, Shengzhong Su, Shipeng Li, Hongkui Liu, Junyou Han and Yaping Yuan * College of Plant Science, Jilin University, Changchun 130062, China; [email protected] (B.L.); [email protected] (X.S.); [email protected] (Y.W.); [email protected] (S.S.); [email protected] (S.L.); [email protected] (H.L.); [email protected] (J.H.) * Correspondence: [email protected] † These authors contributed equally to this work. Received: 13 November 2018; Accepted: 7 December 2018; Published: 12 December 2018 Abstract: Somatic embryos (SE) have potential to rapidly form a whole plant. Generally, SE is thought to be derived from embryogenic calli (EC). However, in maize, not only embryogenic calli (EC, can generate SE) but also nonembryogenic calli (NEC, can’t generate SE) can be induced from immature embryos. In order to understand the differences between EC and NEC and the mechanism of EC, which can easily form SE in maize, differential abundance protein species (DAPS) of EC and NEC from the maize inbred line Y423 were identified by using the isobaric tags for relative and absolute quantification (iTRAQ) proteomic technology. We identified 632 DAPS in EC compared with NEC. The results of bioinformatics analysis showed that EC development might be related to accumulation of pyruvate caused by the DAPS detected in some pathways, such as starch and sucrose metabolism, glycolysis/gluconeogenesis, tricarboxylic acid (TCA) cycle, fatty acid metabolism and phenylpropanoid biosynthesis. -

1 Supplementary Material Figure S1. Volcano Plot of Differentially
Supplementary material Figure S1. Volcano Plot of differentially expressed genes between preterm infants fed own mother’s milk (OMM) or pasteurized donated human milk (DHM). Table S1. The 10 most representative biological processes filtered for enrichment p- value in preterm infants. Biological Processes p-value Quantity of DEG* Transcription, DNA-templated 3.62x10-24 189 Regulation of transcription, DNA-templated 5.34x10-22 188 Transport 3.75x10-17 140 Cell cycle 1.03x10-13 65 Gene expression 3.38x10-10 60 Multicellular organismal development 6.97x10-10 86 1 Protein transport 1.73x10-09 56 Cell division 2.75x10-09 39 Blood coagulation 3.38x10-09 46 DNA repair 8.34x10-09 39 Table S2. Differential genes in transcriptomic analysis of exfoliated epithelial intestinal cells between preterm infants fed own mother’s milk (OMM) and pasteurized donated human milk (DHM). Gene name Gene Symbol p-value Fold-Change (OMM vs. DHM) (OMM vs. DHM) Lactalbumin, alpha LALBA 0.0024 2.92 Casein kappa CSN3 0.0024 2.59 Casein beta CSN2 0.0093 2.13 Cytochrome c oxidase subunit I COX1 0.0263 2.07 Casein alpha s1 CSN1S1 0.0084 1.71 Espin ESPN 0.0008 1.58 MTND2 ND2 0.0138 1.57 Small ubiquitin-like modifier 3 SUMO3 0.0037 1.54 Eukaryotic translation elongation EEF1A1 0.0365 1.53 factor 1 alpha 1 Ribosomal protein L10 RPL10 0.0195 1.52 Keratin associated protein 2-4 KRTAP2-4 0.0019 1.46 Serine peptidase inhibitor, Kunitz SPINT1 0.0007 1.44 type 1 Zinc finger family member 788 ZNF788 0.0000 1.43 Mitochondrial ribosomal protein MRPL38 0.0020 1.41 L38 Diacylglycerol -
![View Using BLAT Program [6] with Default Parameters](https://docslib.b-cdn.net/cover/6947/view-using-blat-program-6-with-default-parameters-8566947.webp)
View Using BLAT Program [6] with Default Parameters
BMC Proceedings BioMed Central Research Open Access Using microarrays to identify positional candidate genes for QTL: the case study of ACTH response in pigs Vincent Jouffe†1, Suzanne Rowe†2, Laurence Liaubet†3, Bart Buitenhuis4, Henrik Hornshøj4, Magali SanCristobal3, Pierre Mormède1 and DJ de Koning*2 Address: 1Laboratoire PsyNuGen, INRA UMR1286, CNRS UMR5226, Université de Bordeaux 2, 146 rue Léo-Saignat, F-33076 Bordeaux, France, 2The Roslin Institute and R(D)SVS, University of Edinburgh, Roslin EH25 9PS, UK, 3Laboratoire de Génétique Cellulaire, INRA UMR444, F-31326 Castanet-Tolosan, France and 4Department of Genetics and Biotechnology, Faculty of Agricultural Sciences, Aarhus University, DK-8830 Tjele, Denmark Email: Vincent Jouffe - [email protected]; Suzanne Rowe - [email protected]; Laurence Liaubet - [email protected]; Bart Buitenhuis - [email protected]; Henrik Hornshøj - [email protected]; Magali SanCristobal - [email protected]; Pierre Mormède - [email protected]; DJ de Koning* - [email protected] * Corresponding author †Equal contributors from EADGENE and SABRE Post-analyses Workshop Lelystad, The Netherlands. 12–14 November 2008 Published: 16 July 2009 BMC Proceedings 2009, 3(Suppl 4):S14 doi:10.1186/1753-6561-3-S4-S14 <supplement> <title> <p>EADGENE and SABRE Post-analyses Workshop</p> </title> <editor>Dirk-Jan de Koning</editor> <sponsor> <note>The publication of these proceedings was supported by the EC-funded Network of Excellence EADGENE (EC contract number FOOD-CT-2004-506416).</note> </sponsor> <note>Proceedings</note> <url>http://www.biomedcentral.com/content/pdf/1753-6561-3-S4-info.pdf</url> </supplement> This article is available from: http://www.biomedcentral.com/1753-6561/3/S4/S14 © 2009 Jouffe et al; licensee BioMed Central Ltd.