Definition of Chalk
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Lithostratigraphy and Biostratigraphy of the Chalk Group (Upper Coniacian 1 to Upper Campanian) at Scratchell’S Bay and Alum Bay, Isle of Wight, UK
Manuscript Click here to view linked References The lithostratigraphy and biostratigraphy of the Chalk Group (Upper Coniacian 1 to Upper Campanian) at Scratchell’s Bay and Alum Bay, Isle of Wight, UK. 2 3 Peter Hopson1*, Andrew Farrant1, Ian Wilkinson1, Mark Woods1 , Sev Kender1 4 2 5 and Sofie Jehle , 6 7 1 British Geological Survey, Sir Kingsley Dunham Centre, Nottingham, NG12 8 5GG. 9 2 10 University of Tübingen, Sigwartstraße 10, 72074 Tübingen, Germany 11 12 * corresponding author [email protected] 13 14 Keywords: Cretaceous, Isle of Wight, Chalk, lithostratigraphy, biostratigraphy, 15 16 17 Abstract 18 19 The Scratchell‟s Bay and southern Alum Bay sections, in the extreme west of the Isle 20 21 of Wight on the Needles promontory, cover the stratigraphically highest Chalk Group 22 formations available in southern England. They are relatively inaccessible, other than 23 by boat, and despite being a virtually unbroken succession they have not received the 24 attention afforded to the Whitecliff GCR (Geological Conservation Review series) 25 site at the eastern extremity of the island. A detailed account of the lithostratigraphy 26 27 of the strata in Scratchell‟s Bay is presented and integrated with macro and micro 28 biostratigraphical results for each formation present. Comparisons are made with 29 earlier work to provide a comprehensive description of the Seaford Chalk, Newhaven 30 Chalk, Culver Chalk and Portsdown Chalk formations for the Needles promontory. 31 32 33 The strata described are correlated with those seen in the Culver Down Cliffs – 34 Whitecliff Bay at the eastern end of the island that form the Whitecliff GCR site. -

Oregon Department of Human Services HEALTH EFFECTS INFORMATION
Oregon Department of Human Services Office of Environmental Public Health (503) 731-4030 Emergency 800 NE Oregon Street #604 (971) 673-0405 Portland, OR 97232-2162 (971) 673-0457 FAX (971) 673-0372 TTY-Nonvoice TECHNICAL BULLETIN HEALTH EFFECTS INFORMATION Prepared by: Department of Human Services ENVIRONMENTAL TOXICOLOGY SECTION Office of Environmental Public Health OCTOBER, 1998 CALCIUM CARBONATE "lime, limewater” For More Information Contact: Environmental Toxicology Section (971) 673-0440 Drinking Water Section (971) 673-0405 Technical Bulletin - Health Effects Information CALCIUM CARBONATE, "lime, limewater@ Page 2 SYNONYMS: Lime, ground limestone, dolomite, sugar lime, oyster shell, coral shell, marble dust, calcite, whiting, marl dust, putty dust CHEMICAL AND PHYSICAL PROPERTIES: - Molecular Formula: CaCO3 - White solid, crystals or powder, may draw moisture from the air and become damp on exposure - Odorless, chalky, flat, sweetish flavor (Do not confuse with "anhydrous lime" which is a special form of calcium hydroxide, an extremely caustic, dangerous product. Direct contact with it is immediately injurious to skin, eyes, intestinal tract and respiratory system.) WHERE DOES CALCIUM CARBONATE COME FROM? Calcium carbonate can be mined from the earth in solid form or it may be extracted from seawater or other brines by industrial processes. Natural shells, bones and chalk are composed predominantly of calcium carbonate. WHAT ARE THE PRINCIPLE USES OF CALCIUM CARBONATE? Calcium carbonate is an important ingredient of many household products. It is used as a whitening agent in paints, soaps, art products, paper, polishes, putty products and cement. It is used as a filler and whitener in many cosmetic products including mouth washes, creams, pastes, powders and lotions. -

Geologic Models and Evaluation of Undiscovered Conventional and Continuous Oil and Gas Resources— Upper Cretaceous Austin Chalk, U.S
Geologic Models and Evaluation of Undiscovered Conventional and Continuous Oil and Gas Resources— Upper Cretaceous Austin Chalk, U.S. Gulf Coast Scientific Investigations Report 2012–5159 U.S. Department of the Interior U.S. Geological Survey Front Cover. Photos taken by Krystal Pearson, U.S. Geological Survey, near the old Sprinkle Road bridge on Little Walnut Creek, Travis County, Texas. Geologic Models and Evaluation of Undiscovered Conventional and Continuous Oil and Gas Resources—Upper Cretaceous Austin Chalk, U.S. Gulf Coast By Krystal Pearson Scientific Investigations Report 2012–5159 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior KEN SALAZAR, Secretary U.S. Geological Survey Marcia K. McNutt, Director U.S. Geological Survey, Reston, Virginia: 2012 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment, visit http://www.usgs.gov or call 1–888–ASK–USGS. For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod To order this and other USGS information products, visit http://store.usgs.gov Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted materials contained within this report. Suggested citation: Pearson, Krystal, 2012, Geologic models and evaluation of undiscovered conventional and continuous oil and gas resources—Upper Cretaceous Austin Chalk, U.S. -
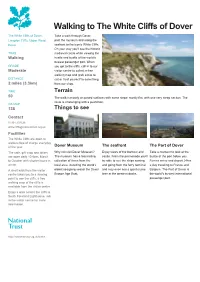
Walking to the White Cliffs of Dover
Walking to The White Cliffs of Dover The White Cliffs of Dover, Take a walk through Dover, Langdon Cliffs, Upper Road, past the museum and along the Dover seafront to the iconic White Cliffs. On your way you'll see the historic TRAIL medieval castle while viewing the Walking hustle and bustle of the world's busiest passenger port. When GRADE you get to the cliffs, call in to our Moderate visitor centre to collect a free walking map and grab a bite to DISTANCE eat or treat yourself to something 2 miles (3.3km) from our shop. TIME Terrain 50 The walk is mostly on paved surfaces with some steps; mostly flat, with one very steep section. The route is challenging with a pushchair. OS MAP 138 Things to see Contact 01304 207326 [email protected] Facilities The White Cliffs are open to walkers free of charge everyday of the year. Dover Museum The seafront The Port of Dover The cafe, gift shop and toilets Why not visit Dover Museum? Enjoy views of the harbour and Take a moment to look at the are open daily 10-5pm, March The museum has a fascinating castle. From the promenade you’ll bustle of the port below you. to October with shorter hours in collection of items from the be able to see the ships coming Ferries arrive and depart 24hrs winter. local area, including the world's and going from the ferry terminal a day traveling to France and A short walk from the visitor oldest seagoing vessel the Dover and may even see a giant cruise Belgium. -

Chemistry of Chalk and Mgco3, Explored Through the Sport of Weightlifting
Teacher Notes: Chemistry of chalk and MgCO3, explored through the sport of weightlifting Sport: Weightlifting Age group: 11 - 14 These notes are designed as a guide on how to lead the session, and are written in a script format. If you wish to lead the session in a different way please feel free to do so. The red text indicates what each slide includes, while the blue text highlights the key points being discussed in each slide. The owl symbol demonstrates where students are required to perform a task (eg questions, experiment, etc.). These are used to help you observe the students learning and recap any information which the students have found difficult to understand. Depending on the level of student understanding this module may require two lessons to complete. Slide 1 Opening slide The first slide provides the title of the session and a picture of a weightlifter dusting chalk containing title of off his hands, introducing the topic and drawing a visual picture of the link between the session and background sport and chemistry. picture as a visual introduction. Slides 2 & 5 – Introduction – Weightlifting and the link to chemistry Weightlifting, the sport of lifting weights in a variety of ways to a variety of different positions, has routes back to ancient times, with the lifting of heavy stones to determine a person’s strength. This has continued through the ages and in many strongman contests today heavy stones are lifted, or attempted to be lifted. Slide 2 gives a small amount of Weightlifting as we know of it today started in Germany in the mid-1800s and quickly background to spread through the rest of Continental Europe. -

Flint and Chert
61 FLINT AND CHERT. By WILLIAM HILL. F.G.S. (Presidential Address, delivered February Srd, 19/1.) CONTE:'l"TS. PAGE I. !)lTRODUCTION. 61 2. T\'PES OF FLINT AND CHERT (CRHACEOUS) 64- 3. VARIETIES OF FLINT FROM FORMATIONS OTHER THAN THE CHALK 69 4. VARIETIES OF CHERT •••" 71 Cherts of the Upper and Lower Greensand. 71 Chert of the Portland Beds. 73 Carboniferous Chert • 76 Chalcedony in Flint . 80 Chert of the Culm Measures 82 5. IMMATURE FLINT AND CHERT 83 6, GENERAL SUMMARY 85 7 CLASSIFICATION OF SILICIOUS CONCRETIONS 93 1. INTRODUCTION. VE RYONE familiar with the White Chalk of England is E also familiar with the nodules of a different substance which are scattered through it. Break one, the fracture will be conchoidal, and it will be found that within a white rind of greater or less thickness is a hard, black, translucent material, possibly with some cloudy patches, which we recognise as flint. How and when the term flint became applied to these nodules in the Chalk is a matter of some obscurity. We know that Early Man recognised the value of flint for offensive, defensive and domestic purposes, and until quite recent years it played no unimportant part in the economies of mankind. The word probably came to us from the Saxon "flinta." It is used six times by the translators of the Authorised Version of the Old and New Testament, though it seems to be applied to rocks of exceptional hardness, and not to be confined to what we know as flint. Virgil, Pliny, and Lucretius use the word silex in connec tion with striking fire. -

08 Feb 2021 Eindrucksvolle Wahrzeichen an Englands Küste
Eindrucksvolle Wahrzeichen an Englands Küste Leuchttürme faszinieren. Sie sind romantisch schön, symbolisieren Weite und Freiheit, sind Landmarken für Schiffe, geben Orientierung und weisen den Weg. Wer sich aus der aktuellen Situation schon einmal „wegträumen“ möchte, wird an Englands Küste leicht fündig und kann interessante Wanderungen häufig von Turm zu Turm planen. „Weit über 60 Leuchttürme reihen sich, einer Perlenschnur gleich, entlang der reizvollen und abwechslungsreichen Küste, viele von ihnen sind der Öffentlichkeit zugänglich“, sagt Samantha Richardson, Direktorin der National Coastal Tourism Academy, die das Projekt „England’s Coast“ leitet. „Die Türme stehen abseits üblicher Touristenpfade, eignen sich wunderbar als Fotomotive und in einigen kann man sogar übernachten – weit weg vom Alltag und inmitten traumhaft einsamer Küstenlandschaften. Und sobald die Einschränkungen es zulassen, machen sie eine Reise an die Küste zu einem magischen Besuch“, so Richardson. England’s Coast ist ein Zusammenschluss aller acht englischen Küstenregionen, die mit dem Projekt ihre Kultur, sagenreiche Geschichte und malerischen Küstenlandschaften vermarkten. Auf Flamborough Head, einer Halbinsel zwischen den Buchten Filey und Bridlington in der Grafschaft Yorkshire, stehen gleich zwei Leuchttürme. Der unter Denkmalschutz stehende Kreideturm aus dem Jahr 1669 ist einer der ältesten vollständig erhaltenen Leuchttürme Englands. Unweit hiervon wurde 1806 ein zweiter Turm erbaut. An den unter Naturschutz stehenden Klippen nisten rund um die Türme über 200.000 Seevögel, darunter Basstölpel und Papageientaucher. Der älteste Leuchtturm des Landes und einer der ältesten der Welt steht auf dem Gelände von Dover Castle. Der Turm wurde um das Jahr 43 n. Chr. von den Römern erbaut, als Navigationshilfe zur Überquerung des Ärmelkanals. Die gut erhaltene Ruine ist ein schönes Fotomotiv und steht direkt neben der kleinen Kirche St. -

Educator Materials (PDF)
Written in Chalk Phenomenal Image Educator Materials HOW TO USE THIS RESOURCE The images for this resource, showing the chalk cliffs of Dover, England, and the microscopic skeletons of coccolithophore algae, can serve as an anchoring phenomenon to explore the key concepts described below. The pedagogical practice of using phenomena to provide a context for understanding science concepts and topics is an implementation practice supported by the Next Generation Science Standards (NGSS). Phenomena are observable occurrences that students can use to generate science questions for further investigation or to design solutions to problems that drive learning. In this way, phenomena connect learning with what is happening in the world while providing students with the opportunity to apply knowledge while they are building it. The “Implementation Suggestions” and “Teaching Tips” sections provide options for incorporating the images into a curriculum or unit of study and can be modified to use as a standalone activity or to supplement an existing lesson. The student handout includes reproductions of the images and the “Background Information” section. KEY CONCEPTS A. Environmental factors such as topography, temperature, and ocean pH can determine the ability of microscopic algae to survive and reproduce over the geologic timescale. B. Specific biological processes, geologic internal processes (tectonic uplift), and surface processes (weathering and erosion) are causal agents in building up and wearing down the Cliffs of Dover over time. C. The geologic record found in the chalk cliffs of Dover can help predict the future effects of climate change on the chemical composition (e.g., ocean pH) and biodiversity of the oceans. -

Uk-England-South-Coast-Self-Drive
Take to the South Coast on this incredible 5-day road trip of England’s seaside towns, from the White Cliffs of Dover to the charming Brighton and the wonderful Isle of Wight. Set out from Heathrow Airport to discover where English soil meets the Channel, encountering the villages and towns that have shaped counties from Kent and Sussex to Hampshire and Dorset. You’ll also get the chance to visit the Isle of Wight and put your feet up in this endearing holiday spot before continuing on to Bournemouth and wider Dorset. dover to dorset 2 | drivenow.com.au – Helping travellers find the best deals on campervan and car rental since 2003 TABLE OF CONTENTS 4 DAY 1: Heathrow - Dover 5 DAY 2: Dover - Rye - Brighton 6 DAY 3: Brighton - Portsmouth - Isle of Wight 7 DAY 4: Isle of Wight - Bournemouth 8 DAY 5: Bournemouth - Dorchester - Weymouth 9 CONCLUSION 3 | drivenow.com.au – Helping travellers find the best deals on campervan and car rental since 2003 day Heathrow - Dover (1 hour, 45 minutes) 1 Finding the right car for your South Coast road trip is easy with DriveNow. Simply select the type of vehicle N you want, pick your dates and let DriveNow do all the NW NE hard work and find you the best deals for your trip. W E This means that when you touch down at Heathrow, SW SE all you have to do is rock on up to your hire car firm, S pick up the keys and jump behind the wheel. You’ll be skirting around the boundary of London on the M25 before following the M20 south to Dover. -

Cultura E Civiltà the White Cliffs of Dover Trascrizione the White Cliffs of Dover Form Part of the Coastline of England Facing
Cultura e Civiltà The White Cliffs of Dover Trascrizione The White Cliffs of Dover form part of the coastline of England facing France. The cliffs are as high as 350 feet in some places. They have a striking appearance because the cliff face is made of chalk with streaks of black flint. Thousands of years ago, the whole area was under the sea. Lots of very small sea creatures died and over the years their bones and shells were pressed together and became chalk. They gradually rose up, becoming white cliffs. Erosion from the seawater keeps the cliffs white and where the sea cannot reach them, they are covered by vegetation. The White Cliffs of Dover are often considered a symbol of England because they are the last sight that travelers see when they leave England, and the first thing they see when they arrive. In fact, at the point where they rise, England is only 21 miles from the French coast. These cliffs were an important part of the defenses of Britain during both World Wars. However, their importance goes much farther back in history. The White Cliffs of Dover stood tall through several invasion attempts, from Julius Caesar in 55 BC to Hitler’s Nazis in 1940. Under the Romans, the Cliffs were used as the base for a lighthouse. In the Middle Ages, Dover Castle, nicknamed ‘The Key to England’ because of its strategic location, was built there. It is the largest castle in England. During the Napoleonic Wars, prisoners of the castle carved secret tunnels under the Cliffs, which Winston Churchill later used as his World War II headquarters. -

Calcium Carbonate
Right to Know Hazardous Substance Fact Sheet Common Name: CALCIUM CARBONATE Synonyms: Calcium Salt of Carbonic Acid, Chalk CAS Number: 1317-65-3 Chemical Name: Limestone RTK Substance Number: 4001 Date: July 2015 DOT Number: NA Description and Use EMERGENCY RESPONDERS >>>> SEE LAST PAGE Calcium Carbonate is a white to tan odorless powder or Hazard Summary odorless crystals. It is used in human medicine as an antacid, Hazard Rating NJDOH NFPA calcium supplement and food additive. Other uses are HEALTH 1 - agricultural lime and as additive in cement, paints, cosmetics, FLAMMABILITY 0 - dentifrices, linoleum, welding rods, and to remove acidity in REACTIVITY 0 - wine. REACTIVE Hazard Rating Key: 0=minimal; 1=slight; 2=moderate; 3=serious; 4=severe Reasons for Citation When Calcium Carbonate is heated to decomposition, it Calcium Carbonate is on the Right to Know Hazardous emits acrid smoke and irritating vapors. Substance List because it is cited by OSHA, NIOSH, and Calcium Carbonate is incompatible with ACIDS, EPA. ALUMINUM, AMMONIUM SALTS, MAGNESIUM, HYDROGEN, FLUORINE and MAGNESIUM. Calcium Carbonate mixed with magnesium and heated in a current of hydrogen causes a violent explosion. Calcium Carbonate ignites on contact with FLUORINE. Calcium Carbonate contact causes irritation to eyes and skin. SEE GLOSSARY ON PAGE 5. Inhaling Calcium Carbonate causes irritation to nose, throat and respiratory system and can cause coughing. FIRST AID Eye Contact Immediately flush with large amounts of water for at least 15 Workplace Exposure Limits minutes, lifting upper and lower lids. Remove contact OSHA: The legal airborne permissible exposure limit (PEL) is lenses, if worn, while flushing. -

NERR080 Edition 1 Natural England Marine Chalk Characterisation Project
Natural England Research Report NERR080 Natural England marine chalk characterisation project www.gov.uk/natural -england Natural England Research Report NERR080 Natural England marine chalk characterisation project Authors: Charlotte Moffat, Heidi Richardson, Georgina Roberts Published March 2020 This report is published by Natural England under the pen Government Licence - OGLv3.0 for public sector information. You are encouraged to use, and reuse, information subject to certain conditions. For details of the licence visit Copyright. Natural England photographs are only available for non commercial purposes. If any other information such as maps or data cannot be used commercially this will be made clear within the report. ISBN 978-1-78354-527-8 © Natural England 2019 Project details This report should be cited as: MOFFAT, C., RICHARDSON, H., ROBERTS, G. 2019. Natural England marine chalk characterisation project. Natural England Research Reports, Number 080. Project manager Charlotte Moffat Marine Lead Adviser Norfolk Coast and Marine Team Natural England Area 5A, Nobel House, 17 Smith Square London SW1P 3JR [email protected] Acknowledgements We would like to thank all those that helped us by providing site specific information, attending the workshop and providing comments on the report. In particular we would like to thank Dr David H Evans for his invaluable help in developing the geology sections. NATURAL ENGLAND MARINE CHALK CHARACTERISATION PROJECT Executive Summary Chalk is a sedimentary rock largely composed of the skeletons of calcareous nanoplankton, deposited between approximately 100 and 65 million years ago during the Late Cretaceous period. Chalk can be heterogenous, with local conditions and the proportion of clay present at time of deposition responsible for the diversity.