Some New Observations on an Old Reaction: Disproportionation and The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Studies on Nucleoside H-Phosphonoselenoate Chemistry and Chalcogen Exchange Reaction Between P(V) and P(III) Compounds
Studies on nucleoside H-phosphonoselenoate chemistry and chalcogen exchange reaction between P(V) and P(III) compounds Martin Kullberg Stockholm University 2005 Doctoral Dissertation Department of Organic Chemistry Arrhenius Laboratory Stockholm University ISBN 91-7155-163-8 Abstract In this thesis, the chemistry of compounds containing P-Se bonds has been studied. As a new addition to this class of compounds, H-phosphonoselenoate monoesters, have been introduced and two synthetic pathways for their preparation have been developed. The reactivity of H-phosphonoselenoate monoesters towards a variety of condensing agents has been studied. From these, efficient conditions for the synthesis of H-phosphonoselenoate diesters have been developed. The produced diesters have subsequently been used in oxidative transformations, which gave access to the corresponding P(V) compounds, e.g. dinucleoside phosphoroselenoates or dinucleoside phosphoroselenothioates. Furthermore, a new selenizing agent, triphenyl phosphoroselenoate, has been developed for selenization of P(III) compounds. This reagent has high solubility in organic solvents and was found to convert phosphite triesters and H-phosphonate diesters efficiently into the corresponding phosphoroselenoate derivatives. The selenization of P(III) compounds with triphenyl phosphoroselenoate proceeds through a selenium transfer reaction. A computational study was performed to gain insight into a mechanism for this reaction. The results indicate that the transfer of selenium or sulfur from P(V) to P(III) compounds proceeds most likely via an X-philic attack of the P(III) nucleophile on the chalcogen of the P(V) species. For the transfer of oxygen, the reaction may also proceed via an edge attack on the P=O bond. Martin Kullberg 2005 Papers are reprinted with permission of the publishers I Table of contents List of Papers III Abbreviations IV 1. -

United States Patent Office Patented Apr
3,803,252 United States Patent Office Patented Apr. 9, 1974 1. 2 3,803,252 in which R is as hereinbefore defined and Y represents PROCESS FOR THE PREPARATION OF CAROTENOD COMPOUNDS a hydrogen atom or a retinyl radical. This formula may Pierre Chabardes, Lyon, and Marc Julia, Paris, France, represent sterically pure products, or mixtures of differ assignors to Rhone-Poulenc S.A., Paris, France ent isomers. 5 The unsubstituted sulphones used as starting mate No Drawing. Filed May 17, 1972, Ser. No. 254,103 rials have the Formula I in which Y represents a hy Claims priority, applicitFrance, May 19, 1971, drogen atom; they are called herein retinyl-Sulphones. Int, C. C07c9 13/00 They can be prepared by known methods for the prep U.S. C. 260-666 C 9 Claims aration of sulphones. A particularly advantageous method O consists of reacting an alkali metal sulphinate of the formula RSOM, in which R represents alkyl, aryl, alkyl ABSTRACT OF THE DISCLOSURE aryl or aralkyl, preferably phenyl or tolyl, and M rep Caroteine compounds e.g. g-caroteine, are prepared by resents an alkali metal, with retinol or a retinol ester reacting a sulphone Ret-SO-R, in which Ret repre of an inorganic or organic acid, such as retinyl chloride sents retinyl or substituted retinyl and R represents a 15 or bromide, or retinyl formate or acetate. They can also hydrocarbon radical, in the presence of an alkaline be prepared by reacting a sulphinic acid RSOH with reagent with an ester Ret-X, in which X represents an retinol, it being possible to form this acid in situ, if re acid residue, and then desulphonating the product, for quired, from a metal sulphinate in an acid medium. -

Triphenyl Phosphite (TPP) EC No 202-908-4 CAS No 101-02-0
Substance Evaluation Conclusion document EC No 202-908-4 SUBSTANCE EVALUATION CONCLUSION as required by REACH Article 48 and EVALUATION REPORT for Triphenyl Phosphite (TPP) EC No 202-908-4 CAS No 101-02-0 Evaluating Member State(s): UK Dated: March 2019 UK CA Page 1 of 101 March 2019 Substance Evaluation Conclusion document EC No 202-908-4 Evaluating Member State Competent Authority UK REACH CA Health and Safety Executive Redgrave Court Merton Road Bootle Merseyside L20 7HS Email:[email protected] Chemicals Assessment Unit Environment Agency Red Kite House, Howbery Park Wallingford Oxfordshire, OX10 8BD Email: [email protected] Year of evaluation in CoRAP: 2013 Before concluding the substance evaluation a Decision to request further information was issued on: 2 December 2015 Further information on registered substances here: http://echa.europa.eu/web/guest/information-on-chemicals/registered-substances UK CA Page 2 of 101 March 2019 Substance Evaluation Conclusion document EC No 202-908-4 DISCLAIMER This document has been prepared by the evaluating Member State as a part of the substance evaluation process under the REACH Regulation (EC) No 1907/2006. The information and views set out in this document are those of the author and do not necessarily reflect the position or opinion of the European Chemicals Agency or other Member States. The Agency does not guarantee the accuracy of the information included in the document. Neither the Agency nor the evaluating Member State nor any person acting on either of their behalves may be held liable for the use which may be made of the information contained therein. -

United States Patent (19) 11 4,311,652 Abramson Et Al
United States Patent (19) 11 4,311,652 Abramson et al. 45 Jan. 19, 1982 54 ARBUZOV REACTIONS EMPLOYING AN 56) References Cited ALEPHATIC SOLVENT U.S. PATENT DOCUMENTS 3,483,279 12/1969 Davis et al. ......................... 260/969 75 Inventors: Alan Abramson, White Plains; 3,699,193 10/1972 Melton ..... ... 260/969 Edward D. Weil, 3,776,984 12/1973 Ratts ................................... 260/969 Hastings-on-Hudson, both of N.Y. Primary Examinter-Anton H. Sutto Attorney, Agent, or Firm-Vivienne T. White 73 Assignee: Stauffer Chemical Company, 57 ABSTRACT Westport, Conn. An improved process for phosphite rearrangement wherein the phosphite is rearranged to the phospho nate. The improvement comprises conducting the phos 21 Appl. No.: 154,170 phite rearrangement reaction in an aliphatic solvent which is miscible with the reactants at reaction temper atures, and immiscible with the product at lower tem 22 Filed: May 28, 1980 peratures. Increased yields of the phosphonate product are obtained without the need for additional distillation 51 int. Cl. ................................................ C07F 9/40 for solvent separation. 52 U.S. C. ................ ... 260/969; 260/990 58 Field of Search ................................ 260/969, 990 12 Claims, No Drawings 4,311,652 3 4. The advantage of conducting the rearrangement in a process for rearranging tris(2-chloroethyl) phosphite. solvent were found to be a more easily controlled exo The invention is directed to phosphite rearrangements, therm due to the heating and reflux of the solvent which wherein the novel process comprises conducting the removes the heat of reaction, and inhibiting the degra rearrangement process in an essentially aliphatic Solvent dation of the phosphonate product produced. -

Synthetic, Sterochemical, and Electronic Studies of Organophosphorous Ligands and Their Transition Metal Complexes James Timothy Spencer Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1984 Synthetic, sterochemical, and electronic studies of organophosphorous ligands and their transition metal complexes James Timothy Spencer Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Inorganic Chemistry Commons Recommended Citation Spencer, James Timothy, "Synthetic, sterochemical, and electronic studies of organophosphorous ligands and their transition metal complexes " (1984). Retrospective Theses and Dissertations. 7727. https://lib.dr.iastate.edu/rtd/7727 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This reproduction was made from a copy of a document sent to us for microfilming. While the most advanced technology has been used to photograph and reproduce this document, the quality of the reproduction is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help clarify markings or notations which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure complete continuity. 2. When an image on the film is obliterated with a round black mark, it is an indication of either blurred copy because of movement during exposure, duplicate copy, or copyrighted materials that should not have been filmed. -

Coordination Chemistry in Liquid Ammonia and Phosphorous Donor Solvents
Coordination Chemistry in Liquid Ammonia and Phosphorous Donor Solvents Kersti B. Nilsson Faculty of Natural Resources and Agricultural Sciences Department of Chemistry Uppsala Doctoral thesis Swedish University of Agricultural Sciences Uppsala 2005 Acta Universitatis Agriculturae Sueciae 2005: number 21 ISSN 1652-6880 ISBN 91-576-7020-x © 2005 Kersti B. Nilsson, Uppsala Tryck: SLU Service/Repro, Uppsala 2005 Abstract Nilsson, K. B., Coordination chemistry in liquid ammonia and phosphorous donor solvents. Doctor’s dissertation. ISSN 1652-6880, ISBN 91-576-7020-x The thesis summarizes and discusses the results from coordination chemistry studies of solvated d10 metal and copper(II) ions, and mercury(II) halide complexes in the strong electron-pair donor solvents liquid and aqueous ammonia, trialkyl phosphite, triphenyl phosphite and trialkylphosphine. The main techniques used are EXAFS, metal NMR and vibrational spectroscopy, and crystallography. Four crystal structures containing ammonia solvated metal ions have been determined. Questions addressed concern whether any changes in the preferential coordination numbers and geometries occur when these metal ions are transferred from aqueous ammonia to liquid ammonia solution, or to phosphorous donor solvents. Liquid ammonia and trialkyl phosphites are found to possess similar electron-pair donor properties, DS=56 for both, while trialkylphosphines are known to be even stronger electron-pair donors. The studies reveal that the ammonia-solvated d10 metal ions obtain very different configurations in liquid ammonia, with gold(I) being linear, copper(I) and silver(I) trigonal, zinc(II) and mercury(II) tetrahedral, and cadmium(II), indium(III) and thallium(III) octahedral. The ammonia-solvated copper(I) and silver(I) ions are linear in aqueous ammonia solution because of the lower ammonia activity, as the third ammine complexes are very weak in aqueous systems. -

Synthesis of 4-Phosphono Β-Lactams and Related Azaheterocyclic Phosphonates
SYNTHESIS OF 4-PHOSPHONO β-LACTAMS AND RELATED AZAHETEROCYCLIC PHOSPHONATES IR . KRISTOF MOONEN To Elza Vercauteren Promotor: Prof. dr. ir. C. Stevens Department of Organic Chemistry, Research Group SynBioC Members of the Examination Committee: Prof. dr. ir. N. De Pauw (Chairman) Prof. dr. J. Marchand-Brynaert Prof. dr. A. Haemers Prof. dr. S. Van Calenbergh Prof. dr. ir. E. Vandamme Prof. dr. ir. R. Verhé Prof. dr. ir. N. De Kimpe Dean: Prof. dr. ir. H. Van Langenhove Rector: Prof. dr. P. Van Cauwenberge IR . KRISTOF MOONEN SYNTHESIS OF 4-PHOSPHONO β-LACTAMS AND RELATED AZAHETEROCYCLIC PHOSPHONATES Thesis submitted in fulfillment of the requirements for the degree of Doctor (PhD) in Applied Biological Sciences: Chemistry Dutch translation of the title: Synthese van 4-fosfono-β-lactamen en aanverwante azaheterocyclische fosfonaten ISBN-Number: 90-5989-129-5 The author and the promotor give the authorisation to consult and to copy parts of this work for personal use only. Every other use is subject to the copyright laws. Permission to reproduce any material contained in this work should be obtained from the author. Woord Vooraf Toen ik op een hete dag in de voorbije zomer dit woord vooraf schreef, stond ik voor één van de laatste horden te nemen in de weg naar het “doctoraat”. Het ideale moment voor een nostalgische terugblik op een zeer fijne periode, hoewel het onzinnig zou zijn te beweren dat alles rozegeur en maneschijn was. En op het einde van de rit komt dan ook het moment waarop je eindelijk een aantal mensen kunt bedanken, omwille van sterk uiteenlopende redenen. -
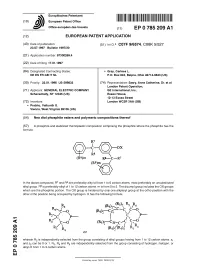
Neo Diol Phosphite Esters and Polymeric Compositions Thereof
^ ^ ^ ^ I ^ ^ ^ ^ ^ ^ II ^ II ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ I ^ European Patent Office Office europeen des brevets EP 0 785 209 A1 EUROPEAN PATENT APPLICATION (43) Date of publication: (51) |nt ci * C07F 9/6574, C08K 5/527 23.07.1997 Bulletin 1997/30 (21) Application number: 97300269.4 (22) Date of filing: 17.01.1997 (84) Designated Contracting States: • Gray, Carloss L. DE ES FR GB IT NL P.O. Box 843, Belpre, Ohio 45714-0843 (US) (30) Priority: 22.01.1996 US 589832 (74) Representative: Szary, Anne Catherine, Dr. et al London Patent Operation, (71) Applicant: GENERAL ELECTRIC COMPANY GE International, Inc., Schenectady, NY 12345 (US) Essex House, 12-13 Essex Street (72) Inventors: London WC2R 3AA (GB) • Prabhu, Vaikunth S. Vienna, West Virginia 26105 (US) (54) Neo diol phosphite esters and polymeric compositions thereof (57) A phosphite and stabilized themoplastic composition comprising the phosphite where the phosphite has the formula: In the above compound, R7 and R8 are preferably alkyl of from 1 to 6 carbon atoms, most preferably an unsubstituted alkyl group. R9 is preferably alkyl of 1 to 1 2 carbon atoms, m is from 0 to 5. The dicumyl group includes the OX groups which are the phosphite portion. The OX group is hindered by only one alkylaryl group at the ortho position with the other ortho position being occupied by hydrogen. X has the following formula: —r'-^C—C C— O ^c— o (Rsk C p- !/C p- *2 2 C— O (R5)r-CZ2-^c^ O) o (R5)/(R5)2' CM or lO 00 wherein R2 is independently selected from the consisting of alkyl having from 1 to 12 carbon atoms, Is- group groups z-, and z2 can be 0 or 1. -

OJC Vol 31(Special
ORIENTAL JOURNAL OF CHEMISTRY ISSN: 0970-020 X CODEN: OJCHEG An International Open Free Access, Peer Reviewed Research Journal 2015, Vol. 31, (Spl Edn): Month : Oct. www.orientjchem.org Pg. 01-12 Aminomethylenephosphonic Acids Syntheses and Applications (A Review) DIDIER VILLEMIN1 and MOHAMED AMINE DIDI2* 1Laboratoire de Chimie Moléculaire et Thioorganique, UMR CNRS 6507, INC3M, FR 3038, Labex EMC3, ENSICAEN, 14050 Caen, France. 2Laboratory of Separation and Purification Technology, Tlemcen University, Faculty of Sciences, Department of Chemistry,Box 119, Algeria. *Corresponding author E-mail: [email protected] (Received: March 25, 2015; Accepted: May 10, 2015) http://dx.doi.org/10.13005/ojc/31.Special-Issue1.01 ABSTRACT A review. In this paper, were reviewed the aminomethylenephosphonic acids which have several synthesis routes and many applications as separation of metals and rare earth by solvant extraction, N-C-P in medicinal chemistry, pesticides/herbicides and water-decontamination systems, organometallic complexes: MOF, gas absorption, proton conductivity and Magnetic shielding tensors / aminotris(methylenephosphonates). Key words: Aminomethylphosphonic; solvent extraction; chelate; rare earth metals; pesticides; herbicides. INTRODUCTION O O H H N C N P Aminophosphonic acid can be an OH OH attractive molecule because it can be used in a OH large range of field. The aim of this report is amino acid aminophosphonic acid to summarize synthesis methods of Fig. 1: Similarity between amino acids aminomethylphosphonic acids and their and aminophosphonic acids applications. Most important characteristic of these This similarity gives to aminomethyl- compounds is their N-C-P molecular fragment that phosphonic acid biological applications in broadly defines them as analogues of amino acids, healthcare and agriculture. -

Federal Register/Vol. 72, No. 191/Wednesday, October 3, 2007
Federal Register / Vol. 72, No. 191 / Wednesday, October 3, 2007 / Notices 56347 B. Tips for Preparing Your Comments. under review and the receipt of notices comment, EPA recommends that you When submitting comments, remember of commencement to manufacture those include your name and other contact to: chemicals. This status report, which information in the body of your • Identify the subject by docket covers the period from August 3, 2007 comment and with any disk or CD ROM number and other identifying to September 7, 2007, consists of the you submit. If EPA cannot read your information (subject heading, Federal PMNs and TMEs, both pending or comment due to technical difficulties Register date and page number). expired, and the notices of and cannot contact you for clarification, • Follow directions—The agency may commencement to manufacture a new EPA may not be able to consider your ask you to respond to specific questions chemical that the Agency has received comment. Electronic files should avoid or organize comments by referencing a under TSCA section 5 during this time the use of special characters, any form Code of Federal Regulations (CFR) part period. of encryption, and be free of any defects or section number. DATES: Comments identified by the or viruses. For additional information • Explain why you agree or disagree; specific PMN number or TME number, about EPA’s public docket, visit the EPA suggest alternatives and substitute must be received on or before November Docket Center homepage at http:// language for your requested changes. 2, 2007. www.epa.gov/epahome/dockets.htm. • Docket: All documents in the docket Describe any assumptions and ADDRESSES: Submit your comments, provide any technical information and/ identified by docket identification (ID) are listed in the docket’s index available or data that you used. -

NMR Studies of Phosphorus Ligand Complexes of Silver and Cobalt Steven Mark Socol Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1983 NMR studies of phosphorus ligand complexes of silver and cobalt Steven Mark Socol Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Inorganic Chemistry Commons Recommended Citation Socol, Steven Mark, "NMR studies of phosphorus ligand complexes of silver and cobalt " (1983). Retrospective Theses and Dissertations. 8962. https://lib.dr.iastate.edu/rtd/8962 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This reproduction was made from a copy of a document sent to us for microfilming. While the most advanced technology has been used to photograph and reproduce this document, the quality of the reproduction is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help clarify markings or notations which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure complete continuity. 2. When an image on the film is obliterated with a round black mark, it is an indication of either blurred copy because of movement during exposure, duplicate copy, or copyrighted materials that should not have been filmed. -

Diaryl Alkylphosphonates and Methods for Preparing
(19) TZZ_Z_T (11) EP 1 940 855 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07F 9/12 (2006.01) C07F 9/145 (2006.01) 01.07.2015 Bulletin 2015/27 C07F 9/40 (2006.01) (21) Application number: 06849055.6 (86) International application number: PCT/US2006/060035 (22) Date of filing: 17.10.2006 (87) International publication number: WO 2007/079272 (12.07.2007 Gazette 2007/28) (54) DIARYL ALKYLPHOSPHONATES AND METHODS FOR PREPARING SAME DIARYL-ALKYLPHOSPHONATE UND HERSTELLUNGSVERFAHREN DAFÜR ALKYLPHOSPHONATES DE DIARYLE ET LEURS MÉTHODES DE SYNTHÈSE (84) Designated Contracting States: • YAO ET AL: "A concise method for the synthesis AT BE BG CH CY CZ DE DK EE ES FI FR GB GR of diaryl aryl- or alkylphosphonates" HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI TETRAHEDRON LETT., vol. 47, 22 November SK TR 2005 (2005-11-22), pages 277-281, XP002495388 Designated Extension States: • DATABASECA [Online] CHEMICAL ABSTRACTS AL BA HR MK RS SERVICE, COLUMBUS, OHIO, US HUDSON, HARRY R. ET AL: ’Quasiphosphonium (30) Priority: 18.10.2005 US 727619 P intermediates. I. Preparation, structure, and 18.10.2005 US 727680 P nuclear magnetic resonance spectroscopy of triphenyl and trineopentyl phosphite- alkyl halide (43) Date of publication of application: adducts’ Retrieved from STN Database 09.07.2008 Bulletin 2008/28 accession no. 1974:449742 & JOURNAL OF THE CHEMICAL SOCIETY, PERKIN TRANSACTIONS (60) Divisional application: 1: ORGANIC AND BIO-ORGANIC CHEMISTRY 11169075.6 / 2 395 011 (1972-1999) , (9), 982-5 CODEN: JCPRB4; ISSN: 0300-922X, 1974, (73) Proprietor: FRX Polymers, Inc.