Investigating Citrulline in Cucurbits: a Survey of Cucurbitaceae and Heritability Estimations in Watermelon (Under the Direction of Todd C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Precios Frutas
Precios de Arribo de Fruta en el Mercado Norteamericano Para obtener la información de los precios de arribo de la fruta en el mercado Norteamericano, siga las siguientes indicaciones. Fuente: USDA Si desea información de precios en Puerto de Arribo (Shipping Point): 1. Selecione fecha (date). 2. Haga click en Shipping Point Si desea información de precios en Terminales Frutícolas (Terminal Market): 1. Seleccione tipo de fruta. 2. Seleccione el puerto de su preferencia (Location), los puertos principales son Los Ángeles y Philadelphia. 3. Selecione fecha (date). 4. Haga click en Shipping Point A - B • Apple Cider • Bananas • Blood Orange • Apple Pears • Batatas • Blueberries • Apples • Bitter Orange • Boysenberries • Apricots • Black Currants • Breadfruit • Avocados • Blackberries C - D • Cactus Pears • Cherimoya • Crenshaw • Cantaloups • Cherries • Dates • Cape Gooseberries • Citron • Dragon Fruit (Red (Physalis) • Clementines Pitaya) • Carambola - Star • Coconuts • Durian Fruit • Cranberries • Casaba • Charantais Melon E - G FreshFruitPortal.com • Edible Flowers • Galia Melon • Grapes-Black Juice • Feijoa • Gooseberries • Grapes-Mixed Juice • Figs • Grapefruit • Grapes-White Juice • Fruits Other • Grapes • Guava H - L • Hami Melon • Kiwano • Limequat • Homelyfruit • Kiwifruit • Limes • Honeydews • Korean Melon • Loganberries • Jackfruit • Kumquats • Longan • Juan Canary Melon • Lee Citrus • Loquat • K-Early Citrus • Lemons • Lychee M - O • Mamey Sapote • Misc Citrus • Orange Flesh Melon • Mangoes • Misc Melons • Oranges • Mangosteen -

Download Catalog
LCTRONICLCTRONIC CATALOGCATALOG Hanging Basket Cucumbers CU20‐20 ‐ Bush Crop Cucumbers CU79‐20 ‐ Mexican Sour Gherkin Cucumbers 55 days. Cucumis sativus. (F1) This early 75 days. Melothria scabra. Open Pollinated. maturing compact bush plant produces high The plant produces heavy yields of 1 to 2" yields of 6 to 8" green cucumbers. They are long cucumbers that look like miniature crisp, tender, and flavorful. Excellent in watermelons. They have the sweet salads or for making dills or fancy sweet cucumber flavor, followed by a surprising pickles. Bush cucumbers produce very nice sourness, which leads you to believe they straight cucumbers. It grows very short are already pickled! Great for Perfect for vines, only 1 to 2 feet long, and is one of the salads, snacks, and pickling. They fall off best varieties for container gardens and the vines when ripe. This cucumber grows small gardens. An excellent choice for home best if grown on trellis or stakes. The most gardens. Disease Resistant: Ccu. cold‐tolerant of all cucumbers and will continue to produce until the first frost. Perfect for container gardening, hanging baskets, or small gardens where space is very limited!. Also known as the Cucamelon and Mouse Melon. An heirloom from Mexico and Central America. CU98‐10 ‐ Salad Bush Cucumbers CU23‐20 ‐ Spacemaster 80 Cucumbers 1988 All‐America Selections Winner! 60 days. Cucumis sativus. Open Pollinated. Compact bush type plant produces heavy 57 days. Cucumis sativus. (F1) This early yields of 7 to 8" long nicely shaped dark maturing compact bushy plant produces green cucumbers. Great for pickling when good yields of 8” long by 2 ¼” wide glossy small, and slicing when they get bigger. -

ORGANIZING COMMITTEE Gerald J. Holmes, Chair, North Carolina State University, Raleigh, North Carolina Jonathan R
ORGANIZING COMMITTEE Gerald J. Holmes, Chair, North Carolina State University, Raleigh, North Carolina Jonathan R. Schultheis, North Carolina State University, Raleigh, North Carolina Todd C. Wehner, North Carolina State University, Raleigh, North Carolina SCIENTIFIC COMMITTEE & EDITORIAL BOARD Angela Davis, USDA-ARS, Lane, Oklahoma, USA Maria Luisa Gomez-Guillamon, Experiment Station ‘La Mayora’, Malaga, SPAIN Elzbieta Kozik, Research Institute of Vegetable Crops, Skierniewice, POLAND Ales Lebeda, Palacky University, Olomouc, CZECH REPUBLIC Donald Maynard, University of Florida, Bradenton, Florida, USA James McCreight, USDA-ARS, Salinas, California, USA Ray Martyn, Purdue University, West Lafayette, Indiana, USA Harry Paris, Newe Ya’ar Research Center, Ramat Yishay, ISRAEL Gordon Rogers, University of Sydney, AUSTRALIA Michel Pitrat, INRA, Montfavet cedex, FRANCE Susan Webb, University of Florida, Gainesville, Florida, USA Xingping Zhang, Syngenta Seeds, Woodland, California, USA SPONSORS Diamond Silver Helena Chemical Bayer CropScience Seminis Vegetable Seed Cerexagri-Nissco Syngenta Chemtura Corporation Dow AgroSciences Hendrix and Dail, Inc. Hollar Seeds North Carolina Department of Agriculture and Consumer Services Valent BioSciences Willhite Seed Inc. Gold Bronze Abbott & Cobb, Inc. Addis Cates Company, Inc. DuPont Clifton Seed Company Harris Moran FMC Corporation Mt. Olive Pickle Company Nat’l Watermelon Promotion Board Nunhems North Carolina Watermelon Assoc. Sakata Seed USA North Carolina Pickle Producers Association ORO Agri Pickle Packers International, Inc. Rupp Seeds, Inc. UAP Carolinas Xenacom PREFACE n behalf of the organizing committee of Cucurbitaceae 2006, I welcome you to Asheville, North Carolina for the 9th biennial O gathering of cucurbitologists. This tradition began in 1989 with Claude Thomas hosting the first conference in South Carolina, U.S.A. -

June Garden Spade
The Garden June 2021 Spade “Reliable, Responsive and Relevant Information for the Missouri Gardener” Value of Plant Tags Subscribe Now! Donna Aufdenberg, MU Extension Field Specialist in Horticulture Receive a monthly email with 573-243-3581, [email protected] a direct link to “The Garden Recently, my family has been talking about rehabbing our front landscape. The Spade”. plants we love, we would like to buy newer/smaller versions. Unfortunately, I SUBSCRIBE HERE!! have discarded the plant tags listing the cultivar that was purchased. You would think that I, a plant enthusiast, would remember each and every plant I placed in the yard, but my memory fails me. Lately, I have been thinking about the virtues of plant tags. All tags are **In This Issue** different. Some tags give little information, while other tell the whole story from Value of Plant Tags 1, 2 common name to planting suggestions. Here is some of the typical information found on a tag. Gardening Calendar 3 Organic Fertilizers 3 A. Common C. name of the plant Kids Ask Dr. Bug 4, 5 E. Every plant has a What is It? 5, 8 D. common name by Town Hall Snippets 6 G. I. which we refer to Monthly Recipe 6 H. it. For example, Common Chickweed 7 F. purple coneflower Book Review 8 (common name) Specialty Melons 9 J. is actually Herbicide Damage 9 Echinaceae Lavender Study 10 A. purpurea How Flowers Got 10 B. (Botanical or scientific name). Their Name Upcoming Events 11 B. Botanical or scientific name - These are in a two name format, genus and species, and are usually found in italics on the tag. -

213 Watermelon and Cantaloupe Variety Trials
WATERMELON AND CANTALOUPE VARIETY TRIALS, 2004 George E. Boyhan1, Darbie Granberry2, Randy Hill3, Thad Paulk4 1East Georgia Extension Center PO Box 8112, GSU Statesboro, GA 30460 [email protected] 2University of Georgia Horticulture Department (Retired) PO Box 1209 Tifton, GA 31793 3Vidalia Onion and Vegetable Research Center 8163 Hwy 178 Lyons, GA 30436 4University of Georgia Horticulture Department PO Box 748 Tifton, GA 31793-0748 Introduction Watermelon and cantaloupe variety trials were conducted at the Vidalia Onion and Vegetable Research Center near Reidsville, GA in Toombs County. These annual trials are to determine suitability of varieties submitted by seed companies for production in Georgia. Methods There were 36 entries in the watermelon trial and seven in the cantaloupe trial. Plants were produced in a local greenhouse seeded on April 7, 2004 and transplanted to the field on May 18, 2004. Fields were prepared according to University of Georgia Cooperative Extension Service recommendations for watermelon and cantaloupe production. Seven hundred fifty pounds of 10-10-10 fertilizer was preplant incorporated and an additional 750 pounds of 10-10-10 was applied approximately four weeks later just prior to vine coverage. Weed control followed University of Georgia Cooperative Extension Service recommendations, however, no disease or insect control measures were taken. The experiments were arranged as randomized complete block designs with four replications. Watermelons were planted with an in-row spacing of 5 ft. and a between row spacing of 6 ft. Each plot (experimental unit) consisted of 10 plants. The cantaloupe experiment had plants set 3 ft in-row with a 6 ft between-row spacing and also had 10 plants per plot. -

Plants and Environment Investigation Report
Plants and environment investigation report Spider establishment The Ministry for Primary Industries’ (MPI) Incursion Investigation prevented (Plants & Environment) and Plant Health Environment Laboratory Spiders found on bagged bulk mail at teams investigate and diagnose suspected exotic pests and NZ Post’s International Mail Centre diseases in the plant and environment sectors. Investigators near Auckland airport were identified as and scientists are based in Auckland and Christchurch. These the false widow spider Steatoda nobilis, teams provide field investigation, diagnostic testing and technical a species not present in New Zealand. expertise with regard to new pests and diseases affecting plants Although this species is not venomous, it and the environment. They also have surveillance and response can inflict a painful bite and is frequently functions and carry out research and development to support confused with the venomous black widow spider. A registered pest control surveillance and incursion response activities. operator visited the facility and found no more of the spiders but applied bendiocarb as a precautionary treatment. Auckland Tamaki campus. This (Guy, 2013). PLMVd was not recorded The mailbags were wrapped in plastic and information became available after the here before 2013 but a 2006 publication incinerated to mitigate any remaining find was posted on the Naturewatch recorded peach calico disease as a biosecurity risk. website. Little published information is “virus-like” disease, based on evidence available on -

Generate Evaluation Form
Great Lakes Fruit, Vegetable & Farm Market EXPO Michigan Greenhouse Growers EXPO December 5-7, 2017 DeVos Place Convention Center, Grand Rapids, MI Vine Crops Where: Grand Gallery (main level) Room E & F MI Recertification credits: 2 (1B, COMM CORE, PRIV CORE) OH Recertification credits: 1 (presentations as marked) CCA Credits: PM(1.0) CM(1.0) Moderator: Ben Phillips, Vegetable Extension Educator, MSU Extension, Saginaw, MI 9:00 am Integrated Management of Powdery Mildew and Gummy Stem Blight on Cucurbits: A Florida Perspective (OH: 2B, 0.5 hr) Gary Vallad, Plant Pathology Dept., Univ. of Florida 9:30 am Perspectives and Opportunities for Growing Orange Flesh and Specialty Melons Jonathan Schultheis, Dept. Horticultural Science, North Carolina State University 10:00 am Best Management Practices: Controlling Insects While Protecting Pollinators (OH: CORE, 0.5 hr) Rick Foster, Entomology Dept., Purdue Univ. 10:30 am Cover Cropping in Melons for Reduced Washing After Harvest Chris Gunter, Horticultural Science Dept., North Carolina State Univ. 11:00 am Session Ends Perspectives and Opportunities for Growing Orange Flesh and Specialty Melons Jonathan R. Schultheis North Carolina State University Department of Horticultural Science 2721 Founders Drive, 264 Kilgore Raleigh, NC 27695-7609 [email protected] Orange Flesh Melon Types: The landscape of orange flesh muskmelons (cantaloupes) has changed dramatically over the past five to ten years. Traditionally, there were two primary orange flesh melon types grown and sold; western types, which are fruits that range in size from about 3 to 5 pounds which are heavily netted and not sutured, and eastern types which have some netting, have a shorter shelf life but have a larger fruit size than a western melon, and tend to have a softer flesh with apparent more flavor than a western melon. -

Plant List 2021-08-25 (12:18)
Plant List 2021-09-24 (14:25) Plant Plant Name Botanical Name in Price Stock Per Unit AFRICAN DREAM ROOT - 1 Silene capensis Yes R92 AFRICAN DREAM ROOT - 2 Silene undulata Yes R92 AFRICAN POTATO Hypoxis hemerocallidea Yes R89 AFRICAN POTATO - SILVER-LEAFED STAR FLOWER Hypoxis rigidula Yes R89 AGASTACHE - GOLDEN JUBILEE Agastache foeniculum No R52 AGASTACHE - HYSSOP, WRINKLED GIANT HYSSOP Agastache rugosa Yes R59 AGASTACHE - LICORICE MINT HYSSOP Agastache rupestris No R59 AGASTACHE - PINK POP Agastache astromontana No R54 AGRIMONY Agrimonia eupatoria No R54 AJWAIN Trachyspermum ammi No R49 ALFALFA Medicago sativa Yes R59 ALOE VERA - ORANGE FLOWER A. barbadensis Yes R59 ALOE VERA - YELLOW FLOWER syn A. barbadensis 'Miller' No R59 AMARANTH - ‘LOVE-LIES-BLEEDING’ Amaranthus caudatus No R49 AMARANTH - CHINESE SPINACH Amaranthus species No R49 AMARANTH - GOLDEN GIANT Amaranthus cruentas No R49 AMARANTH - RED LEAF Amaranthus cruentas No R49 ARTICHOKE - GREEN GLOBE Cynara scolymus Yes R54 ARTICHOKE - JERUSALEM Helianthus tuberosus Yes R64 ARTICHOKE - PURPLE GLOBE Cynara scolymus No R54 ASHWAGANDA, INDIAN GINSENG Withania somniferia Yes R59 ASPARAGUS - GARDEN Asparagus officinalis Yes R54 BALLOON FLOWER - PURPLE Platycodon grandiflorus 'Apoyama' Yes R59 BALLOON FLOWER - WHITE Platycodon grandiflorus var. Albus No R59 BASIL - CAMPHOR Ocimum kilimandscharicum Yes R59 BASIL HOLY - GREEN TULSI, RAM TULSI Ocimum Sanctum Yes R54 BASIL HOLY - TULSI KAPOOR Ocimum sanctum Linn. No R54 BASIL HOLY - TULSI TEMPERATE Ocimum africanum No R54 BASIL HOLY - TULSI -

Origamiinthegarden2 – Interpretation (The Numbers Correspond to the Location on the Exhibit Map.)
OrigamiintheGarden2 – Interpretation (The numbers correspond to the location on the exhibit map.) 1) Inside Out Gallery Butterfly for Jennifer and Kevin Box by Michael G. LaFosse Standing Crane – paper model by Kevin Box Hero’s Horse, Pegasus, Opus #633 by Robert J. Lang Peace Pattern by Kevin Box Pegasus Unfolded, Opus #633 by Kevin Box & Robert J. Lang Butterfly Unfolded, Metamorphosis Mandala by Kevin Box & Michael G. LaFosse Paper Models: Look carefully at each of these three‐dimensional paper forms. Hidden within every folded origami object is a “crease pattern” – a collection of lines forming shapes, which serves as a record of each choice made in creating the object. Can you match the unfolded patterns to their original shapes? What clues help you imagine the crease patterns? Unfolded Wall Hangings: Some origami artists draw or diagram crease patterns as a way to plan a new design or record their steps, developing a simple language of solid and dotted lines to define the difference between mountain and valley folds. As you examine these patterns closely, what do you see? Can you imagine the three‐dimensional forms each pattern creates? 2) Standing Cranes Looming Stars & Botanical Peace2 by Kevin Box These cranes represent the variety of patterns found on origami paper and the many color combinations we see in nature – from richly textured habitats to food and water for living things. But what we perceive transforms as we shift perspective. As you pass through these cranes, look back and reflect: what do you see now? 3) Seed AND Seed Sower Seed by Kevin Box & Beth Johnson Seed Sower by Kevin Box & Michael G. -

Bell Peppers Bull Nose
Bell Peppers Likely introduced to North America in the 1700s. In 1812, Thomas Jefferson recorded Bull Nose Bull Nose (36) peppers in his garden calendar at Monticello. Crisp fruits ripen from green to red with an excellent flavor. Sweet. Productive, sturdy plants. 55-80 days from transplant. Incredibly sweet and delicious, medium-large, 3 or 4-lobed bell peppers mature from green to Chocolate Beauty (36) an attractive chocolate color. Eat them at the fully ripe stage and you'll know that they're something special. Plants are tobacco mosaic virus resistant. Golden Cal Wonder 78 days. Colorful golden bells that are very sweet and tasty. The productive plants produce (72) early and are good for northern climates. 73 days. Plants produce excellent yields of brilliant orange- yellow bell peppers. Blocky, four- Horizon Bell (36) inch fruits are thickwalled, ripening from medium green to orange-yellow at maturity. Sweet and flavorful gourmet pepper for salads, stuffing and more! The best red bell pepper we know for northern gardeners where the seasons are cool and short. King of the North (72) 70 days Large, elongated bells ripen to golden yellow. Plants are very compact, but very productive nonetheless. Tolerates adverse growing conditions. Great choice for low-tunnel growing, early- Napoleon Sweet (36) and late-season, or container planting. Very popular Polish variety that should be a hit over here as well! 75 days Absolutely stunning purple bell pepper. Large 4-lobed, thick-walled fruits borne on sturdy Purple Beauty (36) compact plants. Tender crisp texture, mild sweet flavor. 70-75 days from transplant. -
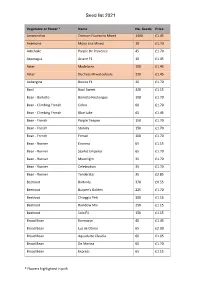
Seed List 2021
Seed list 2021 Vegetable or flower * Name No. Seeds Price Amaranthus Crimson Fountains Mixed 1400 £1.45 Anemone Mona Lisa Mixed 18 £1.70 Artichoke Purple De Provence 45 £1.70 Asparagus Ariane F1 10 £1.45 Aster Madelaine 100 £1.45 Aster Duchess Mixed colours 230 £1.45 Aubergine Bonica F1 20 £1.70 Basil Basil Sweet 320 £1.15 Bean - Borlotto Borlotto Firetongue 100 £1.70 Bean - Climbing French Cobra 60 £1.70 Bean - Climbing French Blue Lake 65 £1.45 Bean - French Purple Teepee 150 £1.70 Bean - French Stanley 150 £1.70 Bean - French Ferrari 100 £1.70 Bean - Runner Enorma 65 £1.15 Bean - Runner Scarlet Emperor 65 £1.70 Bean - Runner Moonlight 35 £1.70 Bean - Runner Celebration 35 £1.70 Bean - Runner Tenderstar 35 £2.85 Beetroot Boltardy 370 £0.55 Beetroot Burpee's Golden 225 £1.70 Beetroot Chioggia Pink 300 £1.15 Beetroot Rainbow Mix 250 £1.15 Beetroot Solo F1 150 £1.15 Broad Bean Karmazyn 40 £1.45 Broad Bean Luz de Otono 65 £2.00 Broad Bean Aquadulce Claudia 60 £1.05 Broad Bean De Monica 60 £1.70 Broad Bean Express 65 £1.15 * Flowers highlighted in pink. Seed list 2021 Vegetable or flower * Name No. Seeds Price Broad Bean Imperial Green Longpod 65 £0.75 Broad Bean The Sutton 60 £0.70 Broccoli Purple Sprouting 400 £0.65 Broccoli Purple Sprouting Continuity Mix 200 £1.45 Broccoli Quarantina 1000 £0.85 Brokali Apollo F1 35 £1.45 Brussel Sprout Continuiity F1 Mix 45 £1.45 Brussels Sprout Brigitte F1 45 £1.45 Brussels Sprout Flower Sprout Mix 30 £1.70 Brussels Sprout Brodie F1 50 £1.70 Brussels Sprout Crispus F1 25 £2.00 Cabbage - Autumn Round -

Spring & Summer 2020
S o u t h e r n H e r i t a g e S e e d C o l l e c t i v e Collection S 2020 Spring & Summer E E D W e a n r d t y b F y r e e it, as it comes up readily on its own! It’s a flowers reliable flower, food, and pollinator plant in Blazing Star our summer gardens, and gets plenty of Liatris tenuifolia attention when visitors tour the gardens. ~100 seeds Grow a bunch one year and you may never A stunning native plant with long 3-6’ spikes of have to get seed again from us! For continuous tufted lavender-colored flower heads. Very harvest, plant every 2-4 weeks attractive to pollinators. Seeds come from Linda Duever of Mockernut Botanical Garden These are traditional greens throughout in Shiloh, Florida. She sows into fresh burns western and central Africa and the most regardless of season, or disturbed soil widely eaten greens in Nigeria. Leaves, tender whenever there is a chance. Nature typically stems, and young flowers can all be used like scatters the seeds November-December spinach. Closely related Callaloo with similar which is the ideal time, but we are distributing growing requirements- easy! Locally saved by these now anyway as spring planting is still ok. SHSC at Grow Hub You can also hold onto these and scatter early fall when cooler temperatures arrive. Do not Cosmos over fertilize or water, these are native plants Cosmos bipinnatus that will actually suffer with too much care! ~45 seeds Locally saved by Linda at Mockernut Hill 52 days.