Seasonal Dynamics of the Fish Assemblage in a Floodplain Lake at the Confluence of the Negro and Amazon Rivers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

'Meeting of Waters'
‘Meeting of waters’ 1 / 4 2 / 4 Released date: Fri, 2019-09-27 12:00 Copyright: contains modified Copernicus Sentinel data (2018), processed by ESA, CC BY-SA 3.0 IGO The Copernicus Sentinel-2 mission takes us over the ‘meeting of waters’ in Brazil – where the Rio Negro and the Solimões River meet to form the Amazon River. Click on the box in the lower-right corner to view this image at its full 10 m resolution directly in your browser. The Rio Negro, visible in black, is the largest tributary of the Amazon and the world’s largest black- water river. It flows 2300 km from Colombia, and it gets its dark colouring from leaf and plant matter that has decayed and dissolved in its waters. The Rio Negro contrasts significantly with the Solimões River – visible directly below - which owes its brown-colouring to its rich sediment content, including sand, mud and silt. After flowing for around 1600 km, the Solimões River meets the Rio Negro and together form this important junction. Owing to differences in temperature, speed and water density, the two rivers, after converging, flow side-by-side for a few kilometres , before eventually mixing. Manaus, the largest city in the Amazon Basin, is visible on the north bank of the Rio Negro. Despite being 1500 km from the ocean, Manaus is a major inland port. The Adolfo Ducke Forest Reserve is visible northeast of the city. The almost square-shaped block of land is a protected area named after the botanist Adolfo Ducke, and is used for the research of biodiversity. -

Clarissa Tossin: Encontro Das Águas (Meeting of Waters)
CLARISSA TOSSIN: ENCONTRO DAS ÁGUAS (MEETING OF WATERS) October 8–November 20, 2016 Meeting of Waters confluence between the dark water of the Rio Negro river and the sandy-colored water of the Rio Solimões with Amazon River in Manaus, Brazil. Photo credit: José Caldas/ BrazilPhotos.com / Alamy Stock Photo. JOAN is pleased to present Clarissa Tossin: Encontro das Águas (Meeting of Waters), a delicate and ambitious large-scale installation. Combining the basket weaving patterns of the indigenous Baniwa people, native to the Brazilian Amazon, with satellite images depicting the 900-miles (1,448 kilometers) of the Amazon River from the Manaus Basin (or Port of Manaus) in Brazil to its equatorial drainage point in the Atlantic Ocean, Tossin will exhibit an enormous tapestry whose cuts and weaves evoke the divide between two systems of representation–cultural and ideological—that exist in Manaus itself. Located within the Amazon rainforest in the Northern region of Brazil, Manaus, the capital city of Amazonas, is situated at the confluence of the Rio Negro (Black River) and the Rio Solimões. The two rivers connect with the Amazon River at the Port of Manaus, where for 3.7 miles (6 kilometers), their nearly black and beige colored waters flow parallel to each other without joining, until the two rivers stream into the Atlantic Ocean. For her exhibition at JOAN, Tossin weaves the cut strips of the tapestry in opposing directions creating visual breaks in the pattern to depict the physical and political fragmentation of the river and its surroundings. This psychic movement mimics the routes of consumer goods, materials, and people in the region. -

Living in Manaus 1. Meet the Melo De Souza Family
Embratur Come and meet two children who live in different parts of the country. See how their lives are similar and different to yours. Living In Manaus With Carlos Melo de Souza, aged 9 This locality study is based around the life of Carlos and his family who live in Brazil. In this study we are taking part in the life of the Melo de Souza family. They live in a booming city called Manaus, which is situated in the developing Northern region of Brazil. The city is surrounded by the Amazon rainforest. 1. Meet the Melo de Souza Family Carlos is nine and lives in a big city called Manaus. It is in the middle of the Amazon Forest. It is the capital of the Brazilian state of Amazonas. The area where he lives is named “Cidade Nova” (New City in Portuguese). It is a new area of the city east of the old centre. Carlos lives with his father, mother and 3 sisters who are 20, 17 and 7 years old. His 11-year- old brother, adopted when he was born, is an Amazonian Indian. Carlos’s father owns a shop. He sells menswear such as shirts and trousers. He travels into Manaus each day by car. It takes him 25 minutes when the traffic is not too busy. THE SOUZA CHILDREN AT HOME 5b. Living in Manaus with Carlos 1 LUNCH BEFORE SCHOOL CARLOS OUTSIDE HIS HOUSE CARLOS’S OWN MAP OF HIS STREET Carlos’s mother does not work but she looks including a bakery called ‘Estivão’, a petrol after the family. -
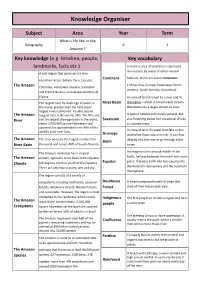
Knowledge Organiser
Knowledge Organiser Subject Area Year Term What is life like in the Geography 6 1 Amazon ? Key knowledge (e.g. timeline, people, Key vocabulary landmarks, facts etc.) A massive area of land that is separated from others by water or other natural A vast region that spans across nine Continent features. There are seven continents: countries: Brazil, Bolivia, Peru, Ecuador, ( Africa, Asia, Europe, Australasia, North The Amazon Colombia, Venezuela, Guyana, Suriname America, South America, Antarctica). and French Guiana, an overseas territory of France. An area of land drained by a river and its The largest river by discharge of water in River Basin tributaries—which is a freshwater stream the world, greater than the next seven that feeds into a larger stream or river. largest rivers combined. It is the second The Amazon longest river in the world, after The Nile and A type of habitat with mostly grasses, but River has the largest drainage basin in the world, Savannah also flowering plants and occasional shrubs about 7,050,000 square kilometers and or isolated trees. accounts for approximately one-fifth of the An area of land. All water that falls on the world's total river flow. Drainage watershed flows into one river. It can flow The Amazon The area home to the largest rainforest in Basin directly into the river or go through tribu- River Basin the world and covers 40% of South America. taries. The Amazon rainforest has a tropical An imaginary line around middle of the The Amazon climate, typical in areas close to the Equator Earth, halfway between the north and south Equator poles. -

The Effects of Small Density Differences at Large Confluences
The effects of small density differences at large confluences Erik van Rooijen i The effects of small density differences at large confluences by Erik van Rooijen 15 November 2016 Graduation Committee: Prof. dr. ir. W.S.J. (Wim) Uijttewaal Delft University of Technology (chairman) Dr. ir. C.J. (Kees) Sloff Delft University of Technology, Deltares (daily supervisor) Dr. ir. E. (Erik) Mosselman Delft University of Technology, Deltares (second reviewer) Dr. Yuan, J. (Jing) National University of Singapore (supervisor) Dr. Chua, V.P. (Vivian) National University of Singapore (examiner) Dr. Ooi, S. (Seng Keat) National University of Singapore (examiner) ii Contents List of symbols ....................................................................................................................................... vii Preface .................................................................................................................................................... 1 Abstract ................................................................................................................................................... 2 1. Introduction .................................................................................................................................... 3 2. Theory and literature ...................................................................................................................... 4 2.1 Main characteristics of confluences ............................................................................................. -

BE BU Amazon Expedition Final 2
Presidente Figueredo Manaus By BUS Operated by: 1504 N Wells Street - 2nd Floor - Chicago, IL - USA 60610 Toll Free: +1 877.BELOUSA - Fax: +1 (312) 376.3690 DAY 1 - FRIDAY MANAUS A group flight will be arranged by Belo USA Travel. The itinerary will be scheduled accordingly. ARRIVAL IN MANAUS: You will arrive in Manaus late Friday evening. Your group will be greeted at the airport by Belo Brasil sta and transported to the hotel. Check-in and have a good night’s rest! *PLANE TICKETS: THE PURCHASE WITH BELO USA IS MANDATORY Please email us at Belo USA Travel at [email protected] and we will search for the best airfare options for your group from your city to Manaus, Brasil. No tickets shall be issued until the trip is confirmed, if you have any questions please contact Belo USA. Icon Legend DAY 2 - SATURDAY MANAUS Arrival Departure Road Trip Breakfast (included). Tour Bike Tour Walking 10:00am - General Orientation Meeting. We will explain the trip rules and discuss the logistics of the tour. Early Departure Sightseeing Relax 12:00pm – The group will start with lunch at a Brazilian mall. Then, we will start our city tour of Manaus with a stop at the local artisanal market where they sell handmade arts and crafts and local medicines. Dinner Show Cave Boat Ride We will then make stops at the historic Downtown area, the famous Opera House, and the riverfront area. After, we’ll return to the hotel and enjoy time the pool. Museum Laundry 7:00 pm - Dinner at the hotel. -

The Upper Rio Negro, Particularly Between Santa Isabel and Sao
UPPER & MIDDLE RIO NEGRO - 9 days Includes Anavilhanas Archipelago and Jau National Park “Cabeça do Cachorro” (“Head of the Dog”), the most untouched native Indian region in Brazil, the frontier of their civilization and entrance of the Guyanas Plateau; many are the titles that recognize the Upper Rio Negro as a “wild” tourist attraction. It is a place with different culture and values that breeds a different kind of man. When you explore the waterways of the upper stretch of one of the world’s oldest rivers, navigating by its islands and beaches, boulder gardens and rapids, riverside mountains, and native communities to which we have privileged access, it is natural that your worldview expands as a result. May different realities be found and great experienced be lived. “Katerre”, which means “good things” in the Yanomami language, certainly will be heard during our expedition until we get to Middle Rio Negro. There you can find Mariuá, the world's largest river archipelago with 700 islands, and many beaches, parrots, and macaws. And, last but not least, the nostalgic town of Barcelos, the first capital of Amazonas. The Upper Rio Negro, particularly between Santa Isabel and Sao Gabriel da Cachoeira, is one of the most beautiful regions of the Amazon with mountains to riverside and unique landscapes. Throughout its complex island formation, there are beaches and large boulders. On good weather days, we see the outline of Pico da Neblina. Anthropologically important, it is the birthplace of Baré, Baniwa Tariano, Tukano and Dessano nations, as well as the greatest empire of the Upper Amazon Indians, the tribe of the Yanomami. -

Birdwatching
Located in northern Brazil, the state of Amazonas has 1.5 million square kilometers, occupying 18% of the country. It borders Colombia, Peru and Venezuela. Its capital, Manaus, has direct international air connections with the United States, Panama, Venezuela and Argentina, as well as daily direct flights to major Brazilian capitals. Holder of the largest freshwater reserve on the planet, water transport is widely used by local people and international cruises. 2 AMAZONAS Largest portion of tropical rainforest on the planet, with 97% preservation rates of native vegetation OPERA HOUSE cover, nature is the major attraction and provides unique experiences. Manaus, the capital, with 350 years of foundation, is a city with 2 million and 100 thousand inhabitants and one of the most important industrial centers of Brazil. It concentrates history and modernity and good surprises to the visitor. 4 SÃO SEBASTIÃO Built in the nineteenth century, is the second most important catholic church in Manaus, in terms of CHURCH building, after the Cathedral of Our Lady of Conception. 5 RIO NEGRO The centennial building was the residence of a “rubber baron” and administrative seat of the State PALACE Government. It currently functions as a cultural CULTURAL center that houses different shows and exhibitions. CENTER 6 MEETING OF THE The phenomenon is the result of the union of the Negro and Solimões rivers, which run kilometers WATERS without mixing due to differences in temperature, density and composition. 7 “BOTO” The red boto or pink boto is a mammal that lives in freshwater. The appearance is very similar to WATCHING dolphins. -

Etho-Ecological Study of the Amazon River Dolphin, Inia
Marine Biodiversity Records, page 1 of 5. # Marine Biological Association of the United Kingdom, 2012 doi:10.1017/S1755267212000176; Vol. 5; e23; 2012 Published online Etho-ecological study of the Amazon River dolphin, Inia geoffrensis (Cetacea: Iniidae), and the dolphins of the genus Sotalia (Cetacea: Delphinidae) in Guama´ River, Amazonia gabriel m.a. dos santos1,2,3, adriano c. quaresma3, rafael r. barata3, bruna m.l. martins1, salvatore siciliano1,4, jose’ de sousa e silva jr1 and renata emin-lima1,5 1Grupo de Estudos de Mamı´feros Aqua´ticos da Amazoˆnia/Museu Paraense Emı´lio Goeldi (GEMAM/MPEG), Coordenac¸a˜ode Zoologia, Setor de Mastozoologia, 2Programa de Po´s-graduac¸a˜o em Zoologia, MPEG/UFPA, 3Universidade Federal do Para´ (UFPA), Instituto de Cieˆncias Biolo´gicas, 4Escola Nacional de Sau´de Pu´blica (ENSP/FIOCRUZ), Grupo de Estudos de Mamı´feros Marinhos da Regia˜o dos Lagos (GEMM-LAGOS), 5Po´s-graduac¸a˜o em Sau´de Pu´blica e Meio Ambiente, ENSP/FIOCRUZ The occurrence of dolphins in the Bele´m area, northern Brazil, has been poorly surveyed. In addition, there has been much speculation on the identity of cetaceans inhabiting these brackish waters. Vantage point observations conducted from September 2008 to October 2010 resulted in the observation of 44 groups of dolphins and 79 individuals. Among these, 56 were Inia geoffrensis (71%) and 23 Sotalia. In addition, 21 boat transects were conducted and recorded 50 individuals in 19 sightings. Among sighted during transects 36 were Sotalia (72%) and 14 were Inia. In both observation methods, Tasco Offshore binoculars were used and size and group composition and behaviour (feeding, resting, socializing, travelling and not identified) were recorded. -

The Sarahu New 08 Macrocosm Wonders of the World (All These Considered Sarahu Nagarazan’S at the Age of 22 to 31)
www.ijcrt.org © 2020 IJCRT | Volume 8, Issue 8 August 2020 | ISSN: 2320-2882 The Sarahu New 08 Macrocosm Wonders of the World (All these considered Sarahu Nagarazan’s at the age of 22 to 31) Sarahu Nagarazan Author, Anthropologist, Sexologist, Physicist, Astronomer, Psychiatrist and Geologist SARAHU GROUP OF COMPANIES AND SARAHU WORLD RECORDS© “The Sarahu New 08 Macrocosm Wonders of the World”, Declared By: Sarahu World Records”, “Sarahu Group of Companies”, “The Sarahu New 07 Human Wonders of the World”, “The Sarahu New 07 Wonders of the World”, “The Sarahu New 06 Universal Wonders of the World”, Sarahu Philosophical Research Administration’ and “Sarahu World Records University and Remaining 12 Organizations”. Wonders Declared Year: 2018/2019 Abstract and Preface: “The_Sarahu_New_08_Macrocosm_World_Wonders_Proclaim” Set and announced the “The New Sarahu 08 Macrocosm wonders of the World." and to continue of the 01st, 02nd, 03rd, 04th, 05th, 06th 07th and 08th Macrocosm Wonders of the world. We are from “Sarahu World Records”, “Sarahu Group of Companies”, “The Sarahu New 07 Wonders of the World”, “The Sarahu New 08 Macrocosm Wonders of the World”, “The Sarahu New 06 Universal Wonders”, Sarahu Philosophical Research Administration’ and “Sarahu World Records University” and remaining 13 organizations. First of all we have done the 1st, 2nd, 3rd, 4th, 5th, 6th 7th and 8th Macrocosm Wonders of the world from Sarahu World Records and the remaining 19 Organizations with 300 manpower and with another audience opinions and recommendations. And we're doing it. Here's how we did and worked on the 08 Macrocosm Wonders of Sarahu. In addition, here is the introduction and function of 19 Organizations websites. -

I Want to You to Tell Me
Fulbright-Hays Summer Seminar Abroad Brazil: Sustainability and the Environment, 2007 A Pororoca of Desire: Genesis, Colonization, Projection, Connection and Sustainability in the Brazilian Amazon Mark Shadle, Professor of English-Writing Eastern Oregon University, La Grande, Oregon Overview: Frogs singing, moths disguised as owls—this project is not a static lesson plan that could be created without a life-changing trip to Brazil. It is a humble response and thank you to all the Fulbright-Hays staff in America and Brazil for the present of a trip to Brazil in the summer of 2007. Think of it more like a love letter written from/to the Amazon. It enacts the pedagogy I developed in Teaching Multiwriting: Researching and Composing with Multiple Genres, Media, Disciplines and Cultures, which lets teachers ask their students to connect personal and academic passions. Some of the Writing/Thinking assignments are from that book, and others are created expressly for this exploration. Most importantly, it’s about helping each other discover questions we care enough about to live, rather than mining answers to questions someone else has asked. This is hypertext, with many supplemental hot links that branch out like the many tributaries of the Amazon: the content becomes the form. Follow the river as it goes up/down, in/out, back/forth, over/under—all toward confluence of a syncretic, associative consciousness of either/and, that will help us move away from being children of the apocalypse. I have chosen to embed other genres in a long narrative poem because it can often more easily hold multiple meanings, and it moves right along, like the current of the Amazon. -

Inspiring Conservation Travel Experiences
Inspiring Conservation Travel Experiences Inspiring Conservation Travel Experiences Welcome to Inspiring Conservation Travel Experiences! Since 2006 Amazon Emotions is committed on offering experiential, conservation-conscious and community-based tourism itineraries. Our vision is to share the wildlife and wilderness areas of Amazon, ensuring they are protected for future generations through awareness and active participation, while changing the lives of those who live here through empowerment initiatives. It is our hope that every adventurer who travels with us will become an active contributor and an Ambassador to protect, conserve and promote the natural heritage which makes the Amazon such a sought after destination. Inspiring Conservation Travel Experiences PRIVATE EXPERIENCES Our Private Experiences blend fun, hands-on exploration, meaningful encounters with people and cultures, and much more free time and choices than other lodges offer —with the option of unique itineraries designed to accommodate just you and the traveling companions of your choice. A nature discovery for all ages! Choose from exciting activities like photography, hiking, monkey tracking, and swimming in a waterfall as you explore the Amazon's tropical wild side enjoying Our Jungle House, an authentic family owned relaxing ecolodge, in the heart of this private rainforest reserve. Select the itinerary below that captures your imagination and pick your travel dates—we’ll take care of the rest. PRIVATE EXPERIENCES AMAZON NATURE EXPERIENCE 3 Days / Year-Round Inspiring Conservation Travel Experiences Activities: Hiking, Monkey Search, Birdwatching, Jungle-to-Table, Night Walk, Overnight Camp in the Rainforest Trip Type : Active Amazon is one of the world’s premier destinations for nature travelers.