Determinants of Systemic Venous Return and the Impact of Positive Pressure Ventilation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blood Volume and Circulation Time in Children
Arch Dis Child: first published as 10.1136/adc.11.61.21 on 1 February 1936. Downloaded from BLOOD VOLUME AND CIRCULATION TIME IN CHILDREN BY H. SECKEL, M.D., Late of the University Children's Clinic, Cologne. This paper is based mainly on the results of the author's own research work on blood volume and circulation time in cases of normal and sick children. The following methods were used:- 1. The colorimetric method for determining the circulating plasma volume, and the haematocrit method for estimating the volume of the total circulating blood; and 2. The histamine rash method for estimating the minimum circulation time of the blood. By means of these two methods there is determined only that portion of the total blood volume which is in rapid circulation, the other part, the so-called stored or depot blood, which is moving slowly or is almost stationary, being neglected. The organs which may act as blood depots are the spleen, the liver, the intestines, the sub-papillary plexus of the skin http://adc.bmj.com/ and possibly the muscles. The greater part of the capillary system of these organs is quite extensive enough to supply stored-up blood as and when required to the more rapid circulation or alternatively, withdraw rapidly circulating blood and store it. This action is regulated by the autonomic nervous system. The circulating blood volume as de-termined by the above methods is not absolutely fixed in quantity but chatnges within wide limits, according to the physiological or pathological conditions under on September 25, 2021 by guest. -

Central Venous Pressure: Uses and Limitations
Central Venous Pressure: Uses and Limitations T. Smith, R. M. Grounds, and A. Rhodes Introduction A key component of the management of the critically ill patient is the optimization of cardiovascular function, including the provision of an adequate circulating volume and the titration of cardiac preload to improve cardiac output. In spite of the appearance of several newer monitoring technologies, central venous pressure (CVP) monitoring remains in common use [1] as an index of circulatory filling and of cardiac preload. In this chapter we will discuss the uses and limitations of this monitor in the critically ill patient. Defining Central Venous Pressure What is the Central Venous Pressure? Central venous pressure is the intravascular pressure in the great thoracic veins, measured relative to atmospheric pressure. It is conventionally measured at the junction of the superior vena cava and the right atrium and provides an estimate of the right atrial pressure. The Central Venous Pressure Waveform The normal CVP exhibits a complex waveform as illustrated in Figure 1. The waveform is described in terms of its components, three ascending ‘waves’ and two descents. The a-wave corresponds to atrial contraction and the x descent to atrial relaxation. The c wave, which punctuates the x descent, is caused by the closure of the tricuspid valve at the start of ventricular systole and the bulging of its leaflets back into the atrium. The v wave is due to continued venous return in the presence of a closed tricuspid valve. The y descent occurs at the end of ventricular systole when the tricuspid valve opens and blood once again flows from the atrium into the ventricle. -

Relationship Between Vasodilatation and Cerebral Blood Flow Increase in Impaired Hemodynamics: a PET Study with the Acetazolamide Test in Cerebrovascular Disease
CLINICAL INVESTIGATIONS Relationship Between Vasodilatation and Cerebral Blood Flow Increase in Impaired Hemodynamics: A PET Study with the Acetazolamide Test in Cerebrovascular Disease Hidehiko Okazawa, MD, PhD1,2; Hiroshi Yamauchi, MD, PhD1; Hiroshi Toyoda, MD, PhD1,2; Kanji Sugimoto, MS1; Yasuhisa Fujibayashi, PhD2; and Yoshiharu Yonekura, MD, PhD2 1PET Unit, Research Institute, Shiga Medical Center, Moriyama, Japan; and 2Biomedical Imaging Research Center, Fukui Medical University, Fukui, Japan Key Words: acetazolamide; cerebrovascular disease; cerebral The changes in cerebral blood flow (CBF) and arterial-to- blood volume; vasodilatory capacity; cerebral perfusion pressure capillary blood volume (V0) induced by acetazolamide (ACZ) are expected to be parallel each other in the normal circula- J Nucl Med 2003; 44:1875–1883 tion; however, it has not been proven that the same changes in those parameters are observed in patients with cerebro- vascular disease. To investigate the relationship between changes in CBF, vasodilatory capacity, and other hemody- namic parameters, the ACZ test was performed after an The ability of autoregulation to maintain the cerebral 15O-gas PET study. Methods: Twenty-two patients with uni- blood flow (CBF), which resides in the cerebral circulation lateral major cerebral arterial occlusive disease underwent despite transient changes in systemic mean arterial blood 15 PET scans using the H2 O bolus method with the ACZ test pressure, has been shown to occur via the mechanism of 15 after the O-gas steady-state method. CBF and V0 for each arteriolar vasodilatation in the cerebral circulation (1). The subject were calculated using the 3-weighted integral vasodilatory change in the cerebral arteries is assumed for method as well as the nonlinear least-squares fitting method. -

Effects of Vasodilation and Arterial Resistance on Cardiac Output Aliya Siddiqui Department of Biotechnology, Chaitanya P.G
& Experim l e ca n i t in a l l C Aliya, J Clinic Experiment Cardiol 2011, 2:11 C f a Journal of Clinical & Experimental o r d l DOI: 10.4172/2155-9880.1000170 i a o n l o r g u y o J Cardiology ISSN: 2155-9880 Review Article Open Access Effects of Vasodilation and Arterial Resistance on Cardiac Output Aliya Siddiqui Department of Biotechnology, Chaitanya P.G. College, Kakatiya University, Warangal, India Abstract Heart is one of the most important organs present in human body which pumps blood throughout the body using blood vessels. With each heartbeat, blood is sent throughout the body, carrying oxygen and nutrients to all the cells in body. The cardiac cycle is the sequence of events that occurs when the heart beats. Blood pressure is maximum during systole, when the heart is pushing and minimum during diastole, when the heart is relaxed. Vasodilation caused by relaxation of smooth muscle cells in arteries causes an increase in blood flow. When blood vessels dilate, the blood flow is increased due to a decrease in vascular resistance. Therefore, dilation of arteries and arterioles leads to an immediate decrease in arterial blood pressure and heart rate. Cardiac output is the amount of blood ejected by the left ventricle in one minute. Cardiac output (CO) is the volume of blood being pumped by the heart, by left ventricle in the time interval of one minute. The effects of vasodilation, how the blood quantity increases and decreases along with the blood flow and the arterial blood flow and resistance on cardiac output is discussed in this reviewArticle. -

Determination of Cardiac Output by Equating Venous Return Curves with Cardiac Response Curves
Determination of Cardiac Output By Equating Ve- nous Return Curves With Cardiac Response Curves1 ARTHUR C. GUYTQN From the Department of Physiology and Biophysics, School of Medicine, University of Mississippi, University, Mississippi HE CONCEPT that the heart responds with increasing cardiac output when there occurs increasing venous return was popularized by Starling and, in- deed, has come to be known as Starling’s law. There are many different forms in which Starling’s law can be expressed,including the relationship of cardiac output to right atria1 pressure, the relationship of cardiac output to the degree of distention of the right ventricle at the end of diastole, the relationship of cardiac work to right atria1 pressure or right ventricular distention, the relationship of left ventricular work to right atria1 pressure or right ventricular distention, etc. For the determina- tion of cardiac output, the form of Starling’s law which will be used in the present discussion is the relationship of cardiac output to mean right atria1 pressure, and this type of cruve will be called the “cardiac responsecurve” to right atria1 pressure. It is well known that many factors in the peripheral circulatory system combine together to determine the rate of venous return to the heart. These include the quantity of blood available, the degree of vascular resistance in various parts of the peripheral circulatory system, and the back pressure from the right atrium. It is with these factors that this paper is especially concerned, and it is hoped that this presentation will demonstrate how cardiac output is determined by equating the peripheral circulatory factors with the cardiac responsecurves. -
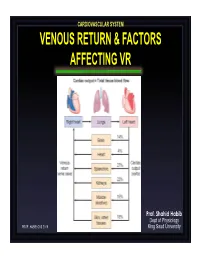
Venous Return & Factors Affecting Vr
CARDIOVASCULAR SYSTEM VENOUS RETURN & FACTORS AFFECTING VR Prof. Shahid Habib Dept of Physiology PROF. HABIB CVS 2019 King Saud University OBJECTIVES At the end of the lecture you should be able to ….. Discuss functions of the veins as blood reservoirs. Describe measurement of central venous pressure (CVP) and state its physiological and clinical significance. State determinants of venous return and explain how they influence venous return. Define mean systemic filling pressure, give its normal value and describe the factors which affect it. Explain the effect of gravity on venous pressure and explain pathophysiology of varicose veins. Describe vascular and cardiac function curves under physiological and pathophysiological conditions. DISTRIBUTION OF BLOOD Capacitance Vessels Veins are blood reservoirs When the body is at rest and many of the capillaries are closed, the capacity of the venous reservoir is increased as extra blood bypasses the capillaries and enters the veins. When this extra volume of blood stretches the veins, the blood moves forward through the veins more slowly because the total cross sectional area of the veins has increased as a result of the stretching. Therefore, blood spends more time in the veins. When the stored blood is needed, such as during exercise, extrinsic factors reduce the capacity of the venous reservoir and drive the extra blood from the veins to the heart so that it can be pumped to the tissues. Rt. Lt. At At Right Left ven ven Large veins Large arteries CAPACITANCE VESSELS EXCHANGE VESSELS RESISTANCE VESSELS Venules Capillaries Arterioles Vascular circuit • all 3 layers are present, but thinner than in •In varicose veins, arteries of corresponding size (external blood pools because diameter). -

Frequency Response of Blood Flow Autoregulation
FREQUENCY RESPONSE OF BLOOD FLOW AUTOREGULATION NICHOLAS BRATTO∗, AFRAH HANEK, AND DAVID WENDL FACULTYADVISORS:DR.HYEJINKIMANDDR.YULIAHRISTOVA INDUSTRYMENTOR:DR.SEBASTIANACOSTA Abstract. Autoregulation is the capability of an organ such as the brain, heart, and kidney to maintain a constant blood flow over a series of changes in arterial pressure within their vascular beds. Since the organs in the humany bod demand a steady delivery of blood and bio–agents to sustain their metabolic activity, autoregulation is crucial in protecting the organs from both over and under perfusion of blood. The impairment of autoregulation may lead to neurological, renal, and other complications. In this study, we analyze a simplified and recently developed mathematical model of blood flow autoregulation based on a system of nonlinear ordinary differential equations. Utilizing this model, we develop the optimal and realistic wall–compliance profiles of the blood vessels. Using the realistic wall–compliance profile, we then findthefrequencyresponseoftheautoregulationsystem. Thefrequencyresponsecanbeusedtodetermine whetheranorganisautoregulatingornotgivensomeinputfrequency. 1. Introduction Autoregulation is an extremely important mechanism that keeps blood flow and volume stable in the circulatory system. The circulatory system regulates the blood flow and supply of nutrients, vitamins, minerals,oxygen,carbondioxide,hormones,metabolicwasteandmoretomeetfunctionalneedsoftissues, muscles,andorgans. Thebasiccomponentsofthesystemconsistsoftheheart,arteries,capillarybeds,and -

Jugular Venous Pressure
NURSING Jugular Venous Pressure: Measuring PRACTICE & SKILL What is Measuring Jugular Venous Pressure? Measuring jugular venous pressure (JVP) is a noninvasive physical examination technique used to indirectly measure central venous pressure(i.e., the pressure of the blood in the superior and inferior vena cava close to the right atrium). It is a part of a complete cardiovascular assessment. (For more information on cardiovascular assessment in adults, see Nursing Practice & Skill ... Physical Assessment: Performing a Cardiovascular Assessment in Adults ) › What: Measuring JVP is a screening mechanism to identify abnormalities in venous return, blood volume, and right heart hemodynamics › How: JVP is determined by measuring the vertical distance between the sternal angle and the highest point of the visible venous pulsation in the internal jugular vein orthe height of the column of blood in the external jugular vein › Where: JVP can be measured in inpatient, outpatient, and residential settings › Who: Nurses, nurse practitioners, physician assistants, and treating clinicians can measure JVP as part of a complete cardiovascular assessment What is the Desired Outcome of Measuring Jugular Venous Pressure? › The desired outcome of measuring JVP is to establish the patient’s JVP within the normal range or for abnormal JVP to be identified so that appropriate treatment may be initiated. Patients’ level of activity should not be affected by having had the JVP measured ICD-9 Why is Measuring Jugular Venous Pressure Important? 89.62 › The JVP is -

Hemodynamic Effects of Pneumonia: II
Hemodynamic effects of pneumonia: II. Expansion of plasma volume Raj Kumar, … , Herbert Benson, Walter H. Abelmann J Clin Invest. 1970;49(4):799-805. https://doi.org/10.1172/JCI106293. Previous work has demonstrated that approximately one-third of patients with pneumonia have a hypodynamic circulatory response. This response is characterized by an abnormally wide arteriovenous oxygen difference, a low cardiac output, increased peripheral resistance, and an increased hematocrit. This state was found to abate in convalescence. In an attempt to elucidate the pathogenesis of this hypodynamic state, nine additional patients were studied hemodynamically during the acute phase of pneumonia before and during acute expansion of blood volume by low molecular weight dextran (seven patients) or normal saline (two patients). Five patients were restudied before and during acute blood volume expansion in convalescence. Three patients with pneumonia had a normal arteriovenous oxygen difference (< 5.5 vol%), and six patients were hypodynamic in that their arteriovenous oxygen differences were greater than 5.5 vol%. With expansion of blood volume in the acute phase of pneumonia, all patients showed an increase in cardiac output, a decrease in arteriovenous oxygen difference, and a decrease in peripheral vascular resistance; however, the percentage change in the hypodynamic patients was not as great as occurred in the patients with normal hemodynamics nor as great as occurred when restudied in convalescence. Likewise, all patients had a normal or near normal hemodynamic profile in convalescence. In addition, ventricular function in the acute phase of pneumonia was depressed. The findings suggest […] Find the latest version: https://jci.me/106293/pdf Hemodynamic Effects of Pneumonia II. -

67 Central Venous/Right Atrial Pressure Monitoring 579
PROCEDURE Central Venous/Right Atrial 67 Pressure Monitoring Reba McVay PURPOSE: Central venous/right-atrial pressure monitoring provides information about the patient ’ s intravascular volume status and right-ventricular preload. The central venous pressure (CVP) or the right atrial pressure (RAP) allows for evaluation of right-sided heart hemodynamics and evaluation of patient response to therapy. CVP and right-atrial pressure are used interchangeably. PREREQUISITE NURSING EQUIPMENT KNOWLEDGE • Pressure transducer system, including fl ush solution rec- • Knowledge of the normal anatomy and physiology of the ommended according to institutional standards, a pressure cardiovascular system is needed. bag or device, pressure tubing with transducer, and fl ush • Knowledge of the principles of aseptic technique and device (see Procedure 75 ) infection control is necessary. • Pressure module and cable for interface with the monitor • Knowledge is needed of the principles of hemodynamic • Dual-channel recorder monitoring. • Leveling device (low-intensity laser or carpenter level) • The CVP/RAP represents right-sided heart preload or the • Nonsterile gloves volume of blood found in the right ventricle at the end of • Sterile injectable or noninjectable caps diastole. Additional equipment (to have available depending on patient • CVP/RAP infl uences and is infl uenced by venous return need) includes the following: and cardiac function. Although the CVP/RAP is used as a • Indelible marker measure of changes in the right ventricle, the relationship is not linear because the right ventricle has the ability to PATIENT AND FAMILY EDUCATION expand and alter its compliance, changes in volume can occur with little change in pressure. • Discuss the purpose of the central venous catheter and • The CVP/RAP normally ranges from 2 to 6 mm Hg in the monitoring with both the patient and family. -

04. the Cardiac Cycle/Wiggers Diagram
Part I Anaesthesia Refresher Course – 2018 4 University of Cape Town The Cardiac Cycle The “Wiggers diagram” Prof. Justiaan Swanevelder Dept of Anaesthesia & Perioperative Medicine University of Cape Town Each cardiac cycle consists of a period of relaxation (diastole) followed by ventricular contraction (systole). During diastole the ventricles are relaxed to allow filling. In systole the right and left ventricles contract, ejecting blood into the pulmonary and systemic circulations respectively. Ventricles The left ventricle pumps blood into the systemic circulation via the aorta. The systemic vascular resistance (SVR) is 5–7 times greater than the pulmonary vascular resistance (PVR). This makes it a high-pressure system (compared with the pulmonary vascular system), which requires a greater mechanical power output from the left ventricle (LV). The free wall of the LV and the interventricular septum form the bulk of the muscle mass in the heart. A normal LV can develop intraventricular pressures up to 300 mmHg. Coronary perfusion to the LV occurs mainly in diastole, when the myocardium is relaxed. The right ventricle receives blood from the venae cavae and coronary circulation, and pumps it via the pulmonary vasculature into the LV. Since PVR is a fraction of SVR, pulmonary arterial pressures are relatively low and the wall thickness of the right ventricle (RV) is much less than that of the LV. The RV thus resembles a passive conduit rather than a pump. Coronary perfusion to the RV occurs continuously during systole and diastole because of the low intraventricular and intramural pressures. In spite of the anatomical differences, the mechanical behaviour of the RV and LV is very similar. -

Blood Vessels and Circulation
19 Blood Vessels and Circulation Lecture Presentation by Lori Garrett © 2018 Pearson Education, Inc. Section 1: Functional Anatomy of Blood Vessels Learning Outcomes 19.1 Distinguish between the pulmonary and systemic circuits, and identify afferent and efferent blood vessels. 19.2 Distinguish among the types of blood vessels on the basis of their structure and function. 19.3 Describe the structures of capillaries and their functions in the exchange of dissolved materials between blood and interstitial fluid. 19.4 Describe the venous system, and indicate the distribution of blood within the cardiovascular system. © 2018 Pearson Education, Inc. Module 19.1: The heart pumps blood, in sequence, through the arteries, capillaries, and veins of the pulmonary and systemic circuits Blood vessels . Blood vessels conduct blood between the heart and peripheral tissues . Arteries (carry blood away from the heart) • Also called efferent vessels . Veins (carry blood to the heart) • Also called afferent vessels . Capillaries (exchange substances between blood and tissues) • Interconnect smallest arteries and smallest veins © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Two circuits 1. Pulmonary circuit • To and from gas exchange surfaces in the lungs 2. Systemic circuit • To and from rest of body © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Circulation pathway through circuits 1. Right atrium (entry chamber) • Collects blood from systemic circuit • To right ventricle to pulmonary circuit 2. Pulmonary circuit • Pulmonary arteries to pulmonary capillaries to pulmonary veins © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Circulation pathway through circuits (continued) 3. Left atrium • Receives blood from pulmonary circuit • To left ventricle to systemic circuit 4.