Accumulation of Heavy Metals in River Sirumugai and the Affect in Human
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coimbatore Commissionerate Jurisdiction
Coimbatore Commissionerate Jurisdiction The jurisdiction of Coimbatore Commissionerate will cover the areas covering the entire Districts of Coimbatore, Nilgiris and the District of Tirupur excluding Dharapuram, Kangeyam taluks and Uthukkuli Firka and Kunnathur Firka of Avinashi Taluk * in the State of Tamil Nadu. *(Uthukkuli Firka and Kunnathur Firka are now known as Uthukkuli Taluk). Location | 617, A.T.D. STR.EE[, RACE COURSE, COIMBATORE: 641018 Divisions under the jurisdiction of Coimbatore Commissionerate Sl.No. Divisions L. Coimbatore I Division 2. Coimbatore II Division 3. Coimbatore III Division 4. Coimbatore IV Division 5. Pollachi Division 6. Tirupur Division 7. Coonoor Division Page 47 of 83 1. Coimbatore I Division of Coimbatore Commissionerate: Location L44L, ELGI Building, Trichy Road, COIMBATORT- 641018 AreascoveringWardNos.l to4,LO to 15, 18to24and76 to79of Coimbatore City Municipal Corporation limit and Jurisdiction Perianaickanpalayam Firka, Chinna Thadagam, 24-Yeerapandi, Pannimadai, Somayampalayam, Goundenpalayam and Nanjundapuram villages of Thudiyalur Firka of Coimbatore North Taluk and Vellamadai of Sarkar Samakulam Firka of Coimbatore North Taluk of Coimbatore District . Name of the Location Jurisdiction Range Areas covering Ward Nos. 10 to 15, 20 to 24, 76 to 79 of Coimbatore Municipal CBE Corporation; revenue villages of I-A Goundenpalayam of Thudiyalur Firka of Coimbatore North Taluk of Coimbatore 5th Floor, AP Arcade, District. Singapore PIaza,333 Areas covering Ward Nos. 1 to 4 , 18 Cross Cut Road, Coimbatore Municipal Coimbatore -641012. and 19 of Corporation; revenue villages of 24- CBE Veerapandi, Somayampalayam, I-B Pannimadai, Nanjundapuram, Chinna Thadagam of Thudiyalur Firka of Coimbatore North Taluk of Coimbatore District. Areas covering revenue villages of Narasimhanaickenpalayam, CBE Kurudampalayam of r-c Periyanaickenpalayam Firka of Coimbatore North Taluk of Coimbatore District. -

Sirumugai Panchayat Solid Waste
1 BEFORE THE NATIONAL GREEN TRIBUNAL SOUTHERN ZONE, CHENNAI Application No.229 of 2016 (SZ) In the matter of M.Gobineelan, No.5/1-I Rest House Road, Sirumugai, Coimbatore 641 302 .. Applicant Vs. 1. The Chairman, Tamil Nadu Pollution Control Board, No.76, Mount Road, Anna Salai, Guindy, Chennai. 2. The District Environmental Engineer, The Tamil Nadu Pollution Control Board, No.266, Mettupalayam Road, Coimbatore North Office, Coimbatore 641 043 3. The District Collector, Coimbatore 4. The Executive Officer, Sirumugai Town Panchayat, Sirumugai Town Panchayat Office, Sirumugai, Mettupalayam, Coimbatore 641 302 .. Respondents Counsel appearing for the appellant M/s N.Stalin & K.Myilsamy Counsel appearing for the Respondent Mr.A.Ilango for R1 & R2 Mr.E.Manoharan for R3 & R4 O R D E R Present Hon’ble Shri Justice M.S.Nambiar, Judicial Member Hon’ble Shri P.S.Rao, Expert Member - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 30th October, 2017 - - - - - - - - - - -- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2 This is an application filed under Section 14 of the National Green Tribunal Act, 2010 for a direction to the 4th respondent to remove the garbage waste and restore the land in Survey No.148/2 and 189 at Ward No.5, Sirumugai Town, Coimbatore District. 2. The case of the applicant is that the 4th respondent is having control over large extent of vacant land in Survey No.148/2 and 189 which is adjacent to the residential house of the applicant and the 4th respondent Executive Officer has started collecting garbage waste through uncovered lorries and other motor vehicles in and around the Sirumugai Town and dumped the garbage waste illegally in the open space causing pollution and therefore there should be a direction to remove the garbage and not to dump or burn the garbage in the land. -

A Local Response to Water Scarcity Dug Well Recharging in Saurashtra, Gujarat
RETHINKING THE MOSAIC RETHINKINGRETHINKING THETHE MOSAICMOSAIC Investigations into Local Water Management Themes from Collaborative Research n Institute of Development Studies, Jaipur n Institute for Social and Environmental Transition, Boulder n Madras Institute of Development Studies, Chennai n Nepal Water Conservation Foundation, Kathmandu n Vikram Sarabhai Centre for Development Interaction, Ahmedabad Edited by Marcus Moench, Elisabeth Caspari and Ajaya Dixit Contributing Authors Paul Appasamy, Sashikant Chopde, Ajaya Dixit, Dipak Gyawali, S. Janakarajan, M. Dinesh Kumar, R. M. Mathur, Marcus Moench, Anjal Prakash, M. S. Rathore, Velayutham Saravanan and Srinivas Mudrakartha RETHINKING THE MOSAIC Investigations into Local Water Management Themes from Collaborative Research n Institute of Development Studies, Jaipur n Institute for Social and Environmental Transition, Boulder n Madras Institute of Development Studies, Chennai n Nepal Water Conservation Foundation, Kathmandu n Vikram Sarabhai Centre for Development Interaction, Ahmedabad Edited by Marcus Moench, Elisabeth Caspari and Ajaya Dixit 1999 1 © Copyright, 1999 Institute of Development Studies (IDS) Institute for Social and Environmental Transition (ISET) Madras Institute of Development Studies (MIDS) Nepal Water Conservation Foundation (NWCF) Vikram Sarabhai Centre for Development Interaction (VIKSAT) No part of this publication may be reproduced nor copied in any form without written permission. Supported by International Development Research Centre (IDRC) Ottawa, Canada and The Ford Foundation, New Delhi, India First Edition: 1000 December, 1999. Price Nepal and India Rs 1000 Foreign US$ 30 Other SAARC countries US$ 25. (Postage charges additional) Published by: Nepal Water Conservation Foundation, Kathmandu, and the Institute for Social and Environmental Transition, Boulder, Colorado, U.S.A. DESIGN AND TYPESETTING GraphicFORMAT, PO Box 38, Naxal, Nepal. -
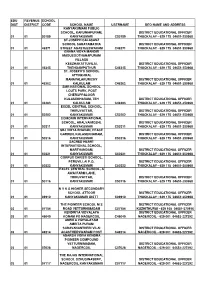
Deo &Ceo Address List
EDU REVENUE SCHOOL DIST DISTRICT CODE SCHOOL NAME USERNAME DEO NAME AND ADDRESS KANYAKUMARI PUBLIC SCHOOL, KARUNIAPURAM, DISTRICT EDUCATIONAL OFFICER 01 01 50189 KANYAKUMARI C50189 THUCKALAY - 629 175 04651-250968 ST.JOSEPH CALASANZ SCHOOL SAHAYAMATHA DISTRICT EDUCATIONAL OFFICER 01 01 46271 STREET AGASTEESWARAM C46271 THUCKALAY - 629 175 04651-250968 GNANA VIDYA MANDIR MADUSOOTHANAPURAM VILLAGE KEEZHAKATTUVILAI, DISTRICT EDUCATIONAL OFFICER 01 01 46345 THENGAMPUTHUR C46345 THUCKALAY - 629 175 04651-250968 ST. JOSEPH'S SCHOOL ATTINKARAI, MANAVALAKURICHY DISTRICT EDUCATIONAL OFFICER 01 01 46362 KALKULAM C46362 THUCKALAY - 629 175 04651-250968 SMR NATIONAL SCHOOL LOUTS PARK, POST CHERUPPALOOR KULASEKHARAM, TEH DISTRICT EDUCATIONAL OFFICER 01 01 46383 KALKULAM C46383 THUCKALAY - 629 175 04651-250968 EXCEL CENTRAL SCHOOL, THIRUVATTAR, DISTRICT EDUCATIONAL OFFICER 01 01 50202 KANYAKUMARI C50202 THUCKALAY - 629 175 04651-250968 COMORIN INTERNATIONAL SCHOOL, ARALVAIMOZHI, DISTRICT EDUCATIONAL OFFICER 01 01 50211 KANYAKUMARI C50211 THUCKALAY - 629 175 04651-250968 SBJ VIDYA BHAVAN, PEACE GARDEN, KULASEKHARAM, DISTRICT EDUCATIONAL OFFICER 01 01 50216 KANYAKUMARI C50216 THUCKALAY - 629 175 04651-250968 SACRED HEART INTERNATIONAL SCHOOL, MARTHANDAM, DISTRICT EDUCATIONAL OFFICER 01 01 50221 KANYAKUMARI C50221 THUCKALAY - 629 175 04651-250968 CORPUS CHRISTI SCHOOL, PERUVILLAI P.O, DISTRICT EDUCATIONAL OFFICER 01 01 50222 KANYAKUMARI C50222 THUCKALAY - 629 175 04651-250968 EXCEL CENTRAL SCHOOL, A AWAI FARM LANE, THIRUVATTAR, DISTRICT EDUCATIONAL OFFICER -

Mettupalayam
METTUPALAYAM S. NO ROLL.NO NAME OF ADVOCATE ADDRESS NO.34D/66, MUNICIPAL COLONY, 1 1340/2010 ABDUL RAZAK M. MANI NAGAR, METTUPALAYAM, COIMBATORE DT. 641301 38/21A, SHAHIB ROWTHAR STREET, ANNAJI RAO ROAD, 2 2398/2011 ABIBOOR RAHMAN I. METTUPALAYAM,COIMBATORE- 641301 NO.5/342-A1, TEACHERS COLONY,KARAMADAI ROAD,OPP 3 1668/2002 ANANDA KUMAR T.A. CTC BUS DEPOT, METTUPALAYAM, COIMBATORE DIST-641301. 2/62,MEECKERY 4 139/1994 ARUN M.B. VILLAGE,B.MANIHATTY POST,THE NILGIRIS-643003 D.NO.4A, UPPUKINATHU PETAI, POLICE SATATION BACKSIDE, 5 351/2007 ASREFFALI A. METTUPALAYAM - 641 301, COIMBATORE DT. NO:5/1135, V.SELVAPURAM NEAR L.E.F.SCHOOL KARAMADAI ROAD 6 2960/2007 BALASUBRAMANI V. METTUPALAYAM COIMBATORE DIST -641301 NO.22/4,NETHAJI 7 993/2006 CHANDRAKUMAR P. NAGAR,KUMARAPURAM METTUPALAYAM-641301 NO. 65, NEW EXTENSIO STREET, 8 66/2007 DHANALASKHMI G. METTUPALAYAM. 42/5, MANIMEKALAI ILLAIM, CHERAN 9 2791/2011 DHANDAPANI P. NAGAR II, METTUPALAYAM 641301 COIMBATORE DIST. NO:4/961, KARUPARAYANPURAM 10 2939/2014 DINESHKUMAR S. ANNUR ROAD METTUPALAYAM COIMBATORE DT -641301. NO:9/177B, VEERASAMY NAGAR 11 1057/1995 GEETHA M. ALANKOMBU METTUPALAYAM TK COIMBATORE -641302 A.42, RAYAN NAGAR, SIRUMUGAI - 641 302 , ILLUPANATHAM VILLAGE, 12 70/1981 GOPOO C.K. METTUPALAYAM TALUK , COIMBATORE DIST . 3/533-D-1, SIRAJ NAGAR, SIRUMUGAI 13 1306/2011 HARI KRISHNAN M. ROAD, METTUPALAYAM 641301 4/595, A.JEEVANANDAPURAM, 14 749/1993 ILANGO R. ANNUR ROAD, METHUPALAYAM, COIMBATORE - 641301. 57/18, APPA NAGAR, OLD SANDAIPET STREET, 15 2266/2012 JAMEESA S. METTUPALAYAM, COIMBATORE DIST - 641 301. VENDADESALU NAGAR, 4TH ST., 16 750/2002 JEEVARAJ S. -

Tamil Nadu Waqf Board
GOVERNMENT OF TAMIL NADU FINAL ELECTORAL ROLL OF MUSLIM MEMBERS OF PARLIAMENT FROM TAMIL NADU 1. Thiru A. Mohammedjan - Rajya Sabha 2. Thiru K. Navaskani - Lok Sabha (Ramanathapuram Constituency) (Sd./-) (Dr.CHANDRA MOHAN. B, I.A.S.,) ELECTION AUTHORITY AND PRINCIPAL SECRETARY TO GOVERNMENT BACKWARD CLASSES, MOST BACKWARD CLASSES AND MINORITIES WELFARE DEPARTMENT, CHENNAI - 9 GOVERNMENT OF TAMIL NADU FINAL ELECTORAL ROLL OF MUSLIM MEMBERS OF STATE LEGISLATIVE ASSEMBLY OF TAMIL NADU Sl. Name of the Member Constituency No. 1. Dr. (Tmt) Nilofer Kafeel Vaniyambadi 2. Thiru M. Thamimun Ansari Nagapattinam 3. Thiru K.S. Masthan Gingee 4. Thiru T.P.M. Mohideen Khan Palayamkottai 5. Thiru K.A.M. Muhammed Abubacker Kadayanallur (Sd./-) (Dr.CHANDRA MOHAN. B, I.A.S.,) ELECTION AUTHORITY AND PRINCIPAL SECRETARY TO GOVERNMENT BACKWARD CLASSES, MOST BACKWARD CLASSES AND MINORITIES WELFARE DEPARTMENT, CHENNAI - 9 GOVERNMENT OF TAMIL NADU FINAL ELECTORAL ROLL OF EX- MUSLIM MEMBERS OF BAR COUNCIL OF TAMIL NADU 1. Thiru M.K. Khan 2. Thiru M. Syed Ismail (Sd./-) (Dr.CHANDRA MOHAN. B, I.A.S.,) ELECTION AUTHORITY AND PRINCIPAL SECRETARY TO GOVERNMENT BACKWARD CLASSES, MOST BACKWARD CLASSES AND MINORITIES WELFARE DEPARTMENT, CHENNAI - 9 ELECTION OF MEMBERS OF TAMIL NADU WAQF BOARD 2020 FINAL ELECTORAL ROLL FOR THE ELECTORAL COLLEGE OF MUTHAWALLIS OF WAQF WHOSE ANNUAL INCOME RUPEES ONE LAKH AND ABOVE 1 S. G.S. NAME OF THE WAQF NAME AND ADDRESS OF ARREARS NO. NO. INSTITUTION MUTHAWALLI / PRESIDENT / OF SECRETARY CONTRI- BUTION As on 31.03.2020 1. 2. 3. 4. 5. CHENNAI DISTRICT 1. GS.3 Ashraf Alisha & Fardalisha V.Syed Jalaludeen, Secretary, 212872 Trust, 1,St.Mary Road, 173,Kutchery Road, Mandaveli,Chennai-28 Mylapore,Chennai-4 2. -

3310 PART a DCHB the NILGIRIS.Pdf
Census of India 2011 TAMIL NADU PART XII-A SERIES-34 DISTRICT CENSUS HANDBOOK THE NILGIRIS VILLAGE AND TOWN DIRECTORY DIRECTORATE OF CENSUS OPERATIONS TAMIL NADU CENSUS OF INDIA 2011 TAMIL NADU SERIES 34 PART XII-A DISTRICT CENSUS HANDBOOK THE NILGIRIS VILLAGE AND TOWN DIRECTORY Directorate of Census Operations Tamil Nadu 2011 THE BOTANICAL GARDEN, OOTY The Botanical Garden is one of the loveliest spot in Udhagamandalam. Started as kitchen garden which was given final shape by the Marquis of Tweeddale in the year 1847. Good maintenance and availability of variety of exotic and ornamental plants bestow on this garden, a unique position among the several gardens in India. Flowers and seedlings are available for sale in the Botanical Garden. There is a wood-hut (Log House) at the top from where panoramic view of Udhagamandalam can be had. The annual Spring Flower Club is held every year during May which attracts large number of tourists. The Botanical Garden has been maintained by the Tamil Nadu Agricultural Department since 1920. In the midst of the garden, just below the small lake, there is a fossil tree trunk of 20 million years old. DISTRICT CENSUS HANDBOOK - 2011 CONTENTS Page Foreword i Preface iii Acknowledgements iv History and Scope of the District Census Handbook v Brief History of the District vi Highlights of the District - 2011 Census viii Important Statistics of the District - 2011 Census ix Analytical Note 1 Village and Town Directory 69 Brief Note on Village and Town Directory 71 Section -I Village Directory 77 (a) List of villages merged in towns and outgrowths at 2011 Census 78 (b) C.D. -

Sl. No. Exporters Name Contact Person Designation Address 1
Sl. Company Products Exporters Name Contact Person Designation Address 1 Address 2 Address 3 Telephone Fax E-mail 1 E-mail 2 E-mail 3 Web ESTD. Exporter Reg. No Registration RCMC Date No. 1 A M K COIR A.M KAMARUDEEN PROPRIETO 47/19, TAMIL INDIA + 91 amkcoirex 2012 Individual Firm Coir Products Merchant P-867 Registration RCMC 06.02.2012 EXPORTS R CHURCH NADU 7598471251 ports@hot (Curled Coir Exporter COMPLEX, 642001 mail.com Rope & Curled POLLACHI, Coir Fibre 2 A N A MOHAMMED MANAGING 42/1399 0477 2239888 +91 anail888@gma 2013 PARTNERSHIP COIR MERCHANT P-889 REGISTRA RCMC 13.06.2013 INTERNATIONAL ISMAIL HASHIM PARTNER CCSB ROAD, 9846139111 il.com FIRM PRODUCTS EXPORTER TION VALID CIVIL VALID UP UP TO STATON TO 31.03.201 WARD, 31.03.2016 8 ALLEPPEY, KERALA - 688001 3 A.J EXPORTS S.RAMESH PROPRIETO 44-B, DD TAMIL +91 rameshsundan 02.01.2013 INDIVIDUAL COIR FIBRE MERCHANT F-664 REGISTRA RCMC 02,05.2013 R MAIN NADU, 9047561644 @gmail.com FIRM EXPORTER TION ROAD, INDIA ARAPALAY AM, MADURAI- 16 4 A.J. COIR A.JAFARULLAH PARTNER S.F.NO.149/2 POLLACHI - INDIA Tel: +91- indianfibr penguinc ESTD.2009 PARTNERSHIP COIR FIBRE MANUFACT F-554 REGISTRA RCMC 22.07.2011 SUPPLIERS C, LAKSHMI 642 006, 9655222786/ e@gmail. oirexport FIRM URER TION NAGAR, TAMIL 9344502525 com @gmail.c EXPORTER RANGASAM NADU om UTHIRAM, 5 A.K BIOSYS A.SUGAVANAM PARTNER V.S.K KOTTAR, TAMIL +91 info@akbi sugavanamak www.akbio ESTD:2009- PRIVATE COIR PITH MERCHANT P-748 REGISTRA RCMC 02.06.2011 COCOPEAT (INDIA) VALAITHOT NAGERCOIL NADU 9442382924 osyscocope @gmail.com syscocopea 2010 COMPANY COIR EXPORTER TION PVT LTD. -
![384] CHENNAI, MONDAY, SEPTEMBER 21, 2020 Purattasi 5, Saarvari, Thiruvalluvar Aandu–2051](https://docslib.b-cdn.net/cover/1908/384-chennai-monday-september-21-2020-purattasi-5-saarvari-thiruvalluvar-aandu-2051-4221908.webp)
384] CHENNAI, MONDAY, SEPTEMBER 21, 2020 Purattasi 5, Saarvari, Thiruvalluvar Aandu–2051
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2020 [Price: Rs. 12.00 Paise. TAMIL NADU GOVERNMENT GAZETTE EXTRAORDINARY PUBLISHED BY AUTHORITY No. 384] CHENNAI, MONDAY, SEPTEMBER 21, 2020 Purattasi 5, Saarvari, Thiruvalluvar Aandu–2051 Part II—Section 2 Notifi cations or Orders of interest to a Section of the public issued by Secretariat Departments. NOTIFICATIONS BY GOVERNMENT REVENUE AND DISASTER MANAGEMENT DEPARTMENT COVID-19 - DEMARCATION OF CONTAINMENT ZONE TO CONTROL CORONA VIRUS - LIST OF CONTAINMENT ZONE AS ON 18TH SEPTEMBER 2020 UNDER THE DISASTER MANAGEMENT ACT, 2005. [G.O. Ms. No. 507, Revenue and Disaster Management (D.M.II), 21st September 2020, ¹ó†ì£C 5, ꣘õK F¼õœÀõ˜ ݇´&2051.] No. II(2)/REVDM/580(b)/2020. The list of Containment Zones as on 18th September 2020 is notifi ed under Disaster Management Act, 2005 for Demarcation of Containment zone to control Corona Virus. Abstract as on 18.09.2020 Sl. No. District No. of Containment Zones (1) (2) (3) 1 Ariyalur 35 2 Chengalpattu 20 3 Coimbatore 100 4 Cuddalore 99 5 Dharmapuri 5 6 Erode 1 7 Kallakurichi 48 8 Kancheepuram 81 9 Kanyakumari 17 10 Karur 2 11 Krishnagiri 46 [1] II-2 Ex. (384)—1 2 TAMIL NADU GOVERNMENT GAZETTE EXTRAORDINARY Sl. No. District No. of Containment Zones (1) (2) (3) 12 Madurai 9 13 Nagapattinam 24 14 Namakkal 25 15 Pudukkottai 29 16 Ramanathapuram 4 17 Ranipet 14 18 Salem 15 19 Sivagangai 2 20 Tenkasi 62 21 Thanjavur 8 22 The Nilgiris 30 23 Theni 106 24 Tiruvarur 51 25 Thoothukudi 9 26 Tiruchirapalli 10 27 Tirunelveli 9 28 Tirupattur 28 29 Tiruppur 71 30 Tiruvallur 29 31 Tiruvannamalai 87 32 Vellore 1 33 Villupuram 15 34 Virudhunagar 7 Total 1099 Chennai, Dindugal and Perambalur - Containment Completed CONTAINMENT ZONES - TAMILNADU - AS ON 18TH SEPTEMBER 2020 Sl. -

111.METTUPALAYAM Assembly Constituency
COIMBATORE DISTRICT 19 THE NILGIRI PARLIMENTARY CONSTITUENCY 111.METTUPALAYAM Assembly Constituency Polling Whether for All Location and name of building in which Part.No station Polling Areas Voters or Men only Polling Station located No. or Women only Panchayat Union Elementary School, 1.Nellithurai (R.V) and (P) Nellithurai Ward No. 1 , ,Nellithurai 641305 2.Nellithurai (R.V) and (P) Nandhavanam Pudur B W.No. 1 1 1 All Voters (East Portion North Facing VI th Std Class Room) 1.Odanthurai (R.V) and (P) Narayanasamy Pudur W.No. 1 , 2.Odanthurai (R.V) and (P) Naripallamroad Mampatti W.No.1 , Sarguru Adivasi Elementary School ,Kallar 3.Odanthurai (R.V) and (P) Ootyroad Puliyamarathuroad 2 2 641305 W.No.1 , 4.Odanthurai (R.V) and (P) All Voters ( East Facing New building, southern Side) Ootyroad Sunnambukalvai W.No.1 , 5.Odanthurai (R.V) and (P) Jallimedu Ooty Road W.No.1 , 6.Odanthurai (R.V) and (P) Kallar Railway Station W.No. 2 , 7.Odanthurai (R.V) and (P) Ooty Road Kallar W.No.2 Sarguru Adivasi Elementary School ,Kallar 3 641305 All Voters (Northern Side, East Facing ) 1.Odanthurai (R.V) and (P) Kallar T.A.S.A Nagar W.No.2 , Panchayat Union Elementary school, 2.Odanthurai (R.V) and (P) Kallar Agasthiyar Nagar W.No.2 Gandhinagar, Odanthurai -641305 ( 4 4 3.Odanthurai (R.V) and (P) Naripallam Road Vinobaji Nagar All Voters North Facing Southern portion RCC Building W.No.2 Tiled Floor) 1.Odanthurai (R.V) and (P) Omaipalayam Killtheru W.No 3,4 Panchayat Union Elementary School, , 5 5 Oomapalayam. -

Action Plan on Rejuvenation of River Bhavani Sirumugai to Kalingarayan Stretch (Priority-IV)
TAMIL NADU POLLUTION CONTROL BOARD Action Plan on Rejuvenation of River Bhavani Sirumugai to Kalingarayan Stretch (Priority-IV) CONTENTS SI. DESCRIPTION PAGE NO. NO. 1.0 Introduction 4 2.0 Introduction about the River Bhavani 7 2.1 Local bodies along the river stretch 8 2.2 Introduction to Polluted River Stretch 10 3.0 Details of inspection team 10 Coimbatore District 4.0 Sources of Pollution in the River Bhavani 11 4.1 River Bhavani - Sewage out-fall points in Coimbatore District 13 4.2 Map showing the River Bhavani – MINARS sampling points 14 4.3 River Bhavani – Sewage out-fall Photographs 15 4.4 Details from Local bodies – Sewage outfall points & Solid waste 16 dumping locations 4.5 Details on Consent / Authorization issued by the Board for the 21 establishment of the STP/ Solid waste facility 4.6 Status on the ground reality of the STPs and Waste processing 21 facilities provided by the local body for handling sewage and solid waste 5.0 Suggestion for improving water quality of the River 22 Erode District 6.0 River Bhavani Stretch Details in Erode Distict 22 7.0 Details of Sewage Outfall Points 22 7.1 River Bhavani – Map Showing Sewage Outfall Points, MINARS & 24 Solid Waste dumping points 7.2 Photographs showing the Sewage outfall Points along the River 25 Bhavani 7.3 Dumping of Solid Wastes into the River or on the Banks with 29 Latitude and Longitude including Photographs 8.0 Status of Sewage Treatment Plant: 30 9.0 Population Details of the local body along the River Stretch 30 10.0 Quantity of water supplied to concerned village -

Coimbatore Sl
COIMBATORE SL. NO. APPLICATION NO. NAME AND ADDRESS ARUNKUMAR.J S/O M.JAYARAJ 1 5517 9/860, KALAINGAR NAGAR, VALPARAI TOWN COVAI, COIMBATORE 642127 SIVAKUMAR. P 33/6, KANNIVADI POST, 2 5518 CHINNADHARA PURAM (VIA), DHARAPURAM TALUK, TIRUPPUR 639202 MUTHURASU. T S/O THIRUMAN. V THANGAMEDU WEST STREET, 3 5519 PUTHUPPAI POST, VELLAKOVIL VIA, DHARAPURAM TK, TIRUPPUR 638111 SARAVANA KUMAR. V S/O GOPAL. A 2/265, 4 5520 S.KUMARAPALAYAM (PO), SULTHAN PET (VIA), SULUR (TALUK), COIMBATORE 641669 RAJAN. K 3/110, VEETHAMPATTY WEST, 5 5521 V.VELUR POST, JAKKAR PALAYAM VIA, MADATHUKULAM TALUK, TIRUPPUR 642202 ANBAZHAGAN.S 18/4, BHAVANI ESTATE, 6 5522 KATTERY POST, COONOOR, NILGIRIS 643213 SATHIYAKEERTHI. R S/O RAJAMANICKAM. I C/O ,YESUDAS, 7 5523 CRAIGMORE TEA FACTORY, KULLAKUMBAY (POST), NILGIRIS 643218 Page 1 SENTHIL KUMAR. R S/O (L) RAMASAMY 152-1, NALLAMMALPURAM, 8 5524 KUTTAPPALAIYAM POST, KANGAYAM TALUK, NATHAKKADAIYUR (VIA), TIRUPPUR 638108 MOORTHY. K S/O KANANDAN .R 2/608, NORTH STREET, 9 5525 PETHIKUTTAI & POST, P.PULIYAMPATTY VIA, COIMBATORE 638459 SARAVANAN. R 6/59A, ANNA NAGAR, 10 5526 KALICKANAICKANPALAYAM, SOUNDAPPALAYAM POST, COIMBATORE 641007 MAHALINGAM. C S/O CHENNIYAPPAN D.NO. 342, EAST STREET, 11 5527 SIVAN MALAI POST, KANGAYAM VIA, TIRUPUR 638701 NAGARAJ. R 66,MGR NAGAR, DHALI ROAD, 12 5528 NEGAMAN, POLLACHI, COIMBATORE 642120 VANITHAMANI . M D/O MANI . V 64,GANDHI PURAM, 13 5529 KATTERY ROAD, COONOR, NILGIRIS 643102 PRAKALATHAN. M 5/195, THODCO COLONY, ML PURAM, 14 5530 CTC MEDU, POLLACHI, COIMBATORE 642002 VINAYAGAMOORTHY. V 92, NANDHANAR COLONY, 15 5531 PALLADAM ROAD, POLLACHI, COIMBATORE 642002 Page 2 ANBAZHAGAN.