The Right Ventricle: Interaction with the Pulmonary Circulation Michael R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 20 *Lecture Powerpoint the Circulatory System: Blood Vessels and Circulation
Chapter 20 *Lecture PowerPoint The Circulatory System: Blood Vessels and Circulation *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • The route taken by the blood after it leaves the heart was a point of much confusion for many centuries – Chinese emperor Huang Ti (2697–2597 BC) believed that blood flowed in a complete circuit around the body and back to the heart – Roman physician Galen (129–c. 199) thought blood flowed back and forth like air; the liver created blood out of nutrients and organs consumed it – English physician William Harvey (1578–1657) did experimentation on circulation in snakes; birth of experimental physiology – After microscope was invented, blood and capillaries were discovered by van Leeuwenhoek and Malpighi 20-2 General Anatomy of the Blood Vessels • Expected Learning Outcomes – Describe the structure of a blood vessel. – Describe the different types of arteries, capillaries, and veins. – Trace the general route usually taken by the blood from the heart and back again. – Describe some variations on this route. 20-3 General Anatomy of the Blood Vessels Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Capillaries Artery: Tunica interna Tunica media Tunica externa Nerve Vein Figure 20.1a (a) 1 mm © The McGraw-Hill Companies, Inc./Dennis Strete, photographer • Arteries carry blood away from heart • Veins -

Central Venous Pressure: Uses and Limitations
Central Venous Pressure: Uses and Limitations T. Smith, R. M. Grounds, and A. Rhodes Introduction A key component of the management of the critically ill patient is the optimization of cardiovascular function, including the provision of an adequate circulating volume and the titration of cardiac preload to improve cardiac output. In spite of the appearance of several newer monitoring technologies, central venous pressure (CVP) monitoring remains in common use [1] as an index of circulatory filling and of cardiac preload. In this chapter we will discuss the uses and limitations of this monitor in the critically ill patient. Defining Central Venous Pressure What is the Central Venous Pressure? Central venous pressure is the intravascular pressure in the great thoracic veins, measured relative to atmospheric pressure. It is conventionally measured at the junction of the superior vena cava and the right atrium and provides an estimate of the right atrial pressure. The Central Venous Pressure Waveform The normal CVP exhibits a complex waveform as illustrated in Figure 1. The waveform is described in terms of its components, three ascending ‘waves’ and two descents. The a-wave corresponds to atrial contraction and the x descent to atrial relaxation. The c wave, which punctuates the x descent, is caused by the closure of the tricuspid valve at the start of ventricular systole and the bulging of its leaflets back into the atrium. The v wave is due to continued venous return in the presence of a closed tricuspid valve. The y descent occurs at the end of ventricular systole when the tricuspid valve opens and blood once again flows from the atrium into the ventricle. -

Young Adults. Look for ST Elevation, Tall QRS Voltage, "Fishhook" Deformity at the J Point, and Prominent T Waves
EKG Abnormalities I. Early repolarization abnormality: A. A normal variant. Early repolarization is most often seen in healthy young adults. Look for ST elevation, tall QRS voltage, "fishhook" deformity at the J point, and prominent T waves. ST segment elevation is maximal in leads with tallest R waves. Note high take off of the ST segment in leads V4-6; the ST elevation in V2-3 is generally seen in most normal ECG's; the ST elevation in V2- 6 is concave upwards, another characteristic of this normal variant. Characteristics’ of early repolarization • notching or slurring of the terminal portion of the QRS wave • symmetric concordant T waves of large amplitude • relative temporal stability • most commonly presents in the precordial leads but often associated with it is less pronounced ST segment elevation in the limb leads To differentiate from anterior MI • the initial part of the ST segment is usually flat or convex upward in AMI • reciprocal ST depression may be present in AMI but not in early repolarization • ST segments in early repolarization are usually <2 mm (but have been reported up to 4 mm) To differentiate from pericarditis • the ST changes are more widespread in pericarditis • the T wave is normal in pericarditis • the ratio of the degree of ST elevation (measured using the PR segment as the baseline) to the height of the T wave is greater than 0.25 in V6 in pericarditis. 1 II. Acute Pericarditis: Stage 1 Pericarditis Changes A. Timing 1. Onset: Day 2-3 2. Duration: Up to 2 weeks B. Findings 1. -

Basic Rhythm Recognition
Electrocardiographic Interpretation Basic Rhythm Recognition William Brady, MD Department of Emergency Medicine Cardiac Rhythms Anatomy of a Rhythm Strip A Review of the Electrical System Intrinsic Pacemakers Cells These cells have property known as “Automaticity”— means they can spontaneously depolarize. Sinus Node Primary pacemaker Fires at a rate of 60-100 bpm AV Junction Fires at a rate of 40-60 bpm Ventricular (Purkinje Fibers) Less than 40 bpm What’s Normal P Wave Atrial Depolarization PR Interval (Normal 0.12-0.20) Beginning of the P to onset of QRS QRS Ventricular Depolarization QRS Interval (Normal <0.10) Period (or length of time) it takes for the ventricles to depolarize The Key to Success… …A systematic approach! Rate Rhythm P Waves PR Interval P and QRS Correlation QRS Rate Pacemaker A rather ill patient……… Very apparent inferolateral STEMI……with less apparent complete heart block RATE . Fast vs Slow . QRS Width Narrow QRS Wide QRS Narrow QRS Wide QRS Tachycardia Tachycardia Bradycardia Bradycardia Regular Irregular Regular Irregular Sinus Brady Idioventricular A-Fib / Flutter Bradycardia w/ BBB Sinus Tach A-Fib VT PVT Junctional 2 AVB / II PSVT A-Flutter SVT aberrant A-Fib 1 AVB 3 AVB A-Flutter MAT 2 AVB / I or II PAT PAT 3 AVB ST PAC / PVC Stability Hypotension / hypoperfusion Altered mental status Chest pain – Coronary ischemic Dyspnea – Pulmonary edema Sinus Rhythm Sinus Rhythm P Wave PR Interval QRS Rate Rhythm Pacemaker Comment . Before . Constant, . Rate 60-100 . Regular . SA Node Upright in each QRS regular . Interval =/< leads I, II, . Look . Interval .12- .10 & III alike .20 Conduction Image reference: Cardionetics/ http://www.cardionetics.com/docs/healthcr/ecg/arrhy/0100_bd.htm Sinus Pause A delay of activation within the atria for a period between 1.7 and 3 seconds A palpitation is likely to be felt by the patient as the sinus beat following the pause may be a heavy beat. -

Effects of Vasodilation and Arterial Resistance on Cardiac Output Aliya Siddiqui Department of Biotechnology, Chaitanya P.G
& Experim l e ca n i t in a l l C Aliya, J Clinic Experiment Cardiol 2011, 2:11 C f a Journal of Clinical & Experimental o r d l DOI: 10.4172/2155-9880.1000170 i a o n l o r g u y o J Cardiology ISSN: 2155-9880 Review Article Open Access Effects of Vasodilation and Arterial Resistance on Cardiac Output Aliya Siddiqui Department of Biotechnology, Chaitanya P.G. College, Kakatiya University, Warangal, India Abstract Heart is one of the most important organs present in human body which pumps blood throughout the body using blood vessels. With each heartbeat, blood is sent throughout the body, carrying oxygen and nutrients to all the cells in body. The cardiac cycle is the sequence of events that occurs when the heart beats. Blood pressure is maximum during systole, when the heart is pushing and minimum during diastole, when the heart is relaxed. Vasodilation caused by relaxation of smooth muscle cells in arteries causes an increase in blood flow. When blood vessels dilate, the blood flow is increased due to a decrease in vascular resistance. Therefore, dilation of arteries and arterioles leads to an immediate decrease in arterial blood pressure and heart rate. Cardiac output is the amount of blood ejected by the left ventricle in one minute. Cardiac output (CO) is the volume of blood being pumped by the heart, by left ventricle in the time interval of one minute. The effects of vasodilation, how the blood quantity increases and decreases along with the blood flow and the arterial blood flow and resistance on cardiac output is discussed in this reviewArticle. -

Basic ECG Interpretation
12/2/2016 Basic Cardiac Anatomy Blood Flow Through the Heart 1. Blood enters right atrium via inferior & superior vena cava 2. Right atrium contracts, sending blood through the tricuspid valve and into the right ventricle 3. Right ventricle contracts, sending blood through the pulmonic valve and to the lungs via the pulmonary artery 4. Re-oxygenated blood is returned to the left atrium via the right and left pulmonary veins 5. Left atrium contracts, sending blood through the mitral valve and into the left ventricle 6. Left ventricle contracts, sending blood through the aortic Septum valve and to the body via the aorta 1 http://commons.wikimedia.org/wiki/File:Diagram_of_the_human_heart 2 _(cropped).svg Fun Fact….. Layers of the Heart Pulmonary Artery – The ONLY artery in the body that carries de-oxygenated blood Pulmonary Vein – The ONLY vein in the body that carries oxygenated blood 3 4 Layers of the Heart Endocardium Lines inner cavities of the heart & covers heart valves (Supplies left ventricle) Continuous with the inner lining of blood vessels Purkinje fibers located here; (electrical conduction system) Myocardium Muscular layer – the pump or workhorse of the heart “Time is Muscle” Epicardium Protective outer layer of heart (Supplies SA node Pericardium in most people) Fluid filled sac surrounding heart 5 6 http://stanfordhospital.org/images/greystone/heartCenter/images/ei_0028.gif 1 12/2/2016 What Makes the Heart Pump? Electrical impulses originating in the right atrium stimulate cardiac muscle contraction Your heart's -

What Is Single Ventricle?
LifeLife withwith aa SingleSingle VentricleVentricle StephenStephen Crumb,Crumb, APNAPN CoordinatorCoordinator CColumbusolumbus OOhiohio AAdultdult CCongenitalongenital HHearteart DiseaseDisease ProgramProgram Biology 101 Q. Your heart has ______ chambers? A. ___3____ Wrong! You need to study!! But you asked how many chambers MY heart has! My heart has only 3 chambers because I have a single ventricle !! ObjectivesObjectives DescribeDescribe thethe congenitalcongenital heartheart defectsdefects thatthat leadlead toto singlesingle ventricleventricle DiscussDiscuss longlong--termterm issuesissues thatthat maymay arisearise inin peoplepeople withwith singlesingle ventricleventricle andand howhow toto treattreat themthem DiscussDiscuss waysways toto staystay healthyhealthy LearnLearn fromfrom eacheach otherother TheThe NormalNormal HeartHeart WhatWhat isis SingleSingle Ventricle?Ventricle? AbnormalAbnormal heartheart structurestructure presentpresent atat birthbirth OnlyOnly oneone functionalfunctional pumpingpumping chamberchamber (ventricle)(ventricle) SeveralSeveral congenitalcongenital heartheart defectsdefects leadlead toto singlesingle ventricle,ventricle, aa featurefeature ofof allall isis cyanosiscyanosis CyanosisCyanosis BlueBlue colorcolor duedue toto lowlow bloodblood oxygenoxygen CausedCaused byby mixingmixing ofof oxygenoxygen--poorpoor (blue)(blue) bloodblood andand oxygenoxygen--richrich (red)(red) bloodblood MixingMixing isis necessarynecessary forfor initialinitial survivalsurvival HypoplasticHypoplastic LeftLeft -

Visualizing Real Tim E Vasomotion in Vivo Using Optical Coherence
A Visualizing real time vasomotion in vivo using Optical Coherence Tom ography Suhir Abuhajar 2012 Lancaster University-Physics Department-Nonlinear Biomedical Physics Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy in Physics ProQuest Number: 11003436 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 11003436 Published by ProQuest LLC(2018). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 4 8 1 0 6 - 1346 C ontents Declaration v Abstract vi Acknowledgment vii 1 Introduction 1 1.1 Motivation .................................................................................................... 1 1.2 Structure of the th esis ............................................................................. 2 1.3 Physiology of the skin ............................................................................. 5 1.3.1 Functions of the s k i n ................................................................... 7 1.3.2 Structure of human s k i n ............................................................ 9 1.3.3 -
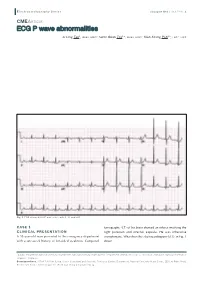
ECG P Wave Abnormalities
E lectrocardiography S eries Singapore Med J 2013; 54(1) : 4 CMEARTICLE ECG P wave abnormalities Li Ling Tan1, MBBS, MRCP, Swee Guan Teo1,2, MBBS, MRCP, Kian Keong Poh1,2, FRCP, FACC Fig. 1 ECG shows bifid P waves in leads II, III and aVF. CASE 1 tomography (CT) of his brain showed an infarct involving the CLINICAL PRESENTATION right putamen and internal capsule. He was otherwise A 58-year-old man presented to the emergency department asymptomatic. What does the electrocardiogram (ECG) in Fig. 1 with a one-week history of left-sided weakness. Computed show? 1Cardiac Department, National University Heart Centre, National University Health System, 2Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore Correspondence: A/Prof Poh Kian Keong, Senior Consultant and Associate Professor, Cardiac Department, National University Heart Centre, 1E Kent Ridge Road, NUHS Tower Block, Level 9, Singapore 119228. [email protected] E lectrocardiography S eries 2a 2b Fig. 2 (a) Echocardiogram in the parasternal long axis view shows a calcified mitral valve. There is restricted opening and closing of the posterior mitral valve leaflet (thick arrow), as well as doming of the anterior mitral valve leaflet (thin arrow); (b) At diastole, the mitral valve opening is markedly reduced (outlined and labeled with an asterisk). RV: right ventricle; LV: left ventricle; LA: left atrium ECG INTERPRETATION CLINICAL COURSE ECG shows the presence of ‘P mitrale’ in multiple leads – inferior Transthoracic echocardiogram showed moderately severe mitral leads II, III and aVF, and also leads V2, V3 and V4. The duration stenosis (Fig. -

Clinical Assessment in Acute Heart Failure
Hellenic J Cardiol 2015; 56: 285-301 Review Article Clinical Assessment in Acute Heart Failure 1 2 NIKOLAOS S. KAKOUROS , STAVROS N. KAKOUROS 1University of Massachusetts, MA, USA; 2Cardiac Department, “Amalia Fleming” General Hospital, Athens, Greece Key words: eart failure (HF) is defined as “a clear precipitant or trigger. It is very im Heart failure, complex clinical syn drome that portant to establish the precipitating diagnosis, physical examination, H can result from any structural or causes, which may have therapeutic and congestion. functional cardiac disorder that impairs the prognostic implications. Approximate ability of the ventricle to fill with, or eject ly 60% of patients with AHF have doc blood.” HF has an estimated overall prev umented CAD. Myocardial ischemia in alence of 2.6%. It is becoming more com the setting of acute coronary syndromes mon in adults older than 65 years, because is a precipitant or cause, particularly in of increased survival after acute myocar patients presenting with de novo AHF.4 dial infarction (AMI) and improved treat AHF is also often precipitated by medica ment of coronary artery disease (CAD), tion and dietary noncompliance, as well val vular heart disease and hypertension.1 as by many other conditions, which are Acute HF (AHF) is an increasingly com summarized in Table 1. Once the diagno mon cause of hospitalizations and mortality sis of AHF is confirmed, initial therapy in worldwide. In the majority of patients, AHF cludes removal of precipitants; if this can Manuscript received: can be attributed to worsening chronic HF, be carried out successfully, the patient’s August 25, 2014; and approximately 4050% of this group have subsequent course may be stable. -

67 Central Venous/Right Atrial Pressure Monitoring 579
PROCEDURE Central Venous/Right Atrial 67 Pressure Monitoring Reba McVay PURPOSE: Central venous/right-atrial pressure monitoring provides information about the patient ’ s intravascular volume status and right-ventricular preload. The central venous pressure (CVP) or the right atrial pressure (RAP) allows for evaluation of right-sided heart hemodynamics and evaluation of patient response to therapy. CVP and right-atrial pressure are used interchangeably. PREREQUISITE NURSING EQUIPMENT KNOWLEDGE • Pressure transducer system, including fl ush solution rec- • Knowledge of the normal anatomy and physiology of the ommended according to institutional standards, a pressure cardiovascular system is needed. bag or device, pressure tubing with transducer, and fl ush • Knowledge of the principles of aseptic technique and device (see Procedure 75 ) infection control is necessary. • Pressure module and cable for interface with the monitor • Knowledge is needed of the principles of hemodynamic • Dual-channel recorder monitoring. • Leveling device (low-intensity laser or carpenter level) • The CVP/RAP represents right-sided heart preload or the • Nonsterile gloves volume of blood found in the right ventricle at the end of • Sterile injectable or noninjectable caps diastole. Additional equipment (to have available depending on patient • CVP/RAP infl uences and is infl uenced by venous return need) includes the following: and cardiac function. Although the CVP/RAP is used as a • Indelible marker measure of changes in the right ventricle, the relationship is not linear because the right ventricle has the ability to PATIENT AND FAMILY EDUCATION expand and alter its compliance, changes in volume can occur with little change in pressure. • Discuss the purpose of the central venous catheter and • The CVP/RAP normally ranges from 2 to 6 mm Hg in the monitoring with both the patient and family. -

04. the Cardiac Cycle/Wiggers Diagram
Part I Anaesthesia Refresher Course – 2018 4 University of Cape Town The Cardiac Cycle The “Wiggers diagram” Prof. Justiaan Swanevelder Dept of Anaesthesia & Perioperative Medicine University of Cape Town Each cardiac cycle consists of a period of relaxation (diastole) followed by ventricular contraction (systole). During diastole the ventricles are relaxed to allow filling. In systole the right and left ventricles contract, ejecting blood into the pulmonary and systemic circulations respectively. Ventricles The left ventricle pumps blood into the systemic circulation via the aorta. The systemic vascular resistance (SVR) is 5–7 times greater than the pulmonary vascular resistance (PVR). This makes it a high-pressure system (compared with the pulmonary vascular system), which requires a greater mechanical power output from the left ventricle (LV). The free wall of the LV and the interventricular septum form the bulk of the muscle mass in the heart. A normal LV can develop intraventricular pressures up to 300 mmHg. Coronary perfusion to the LV occurs mainly in diastole, when the myocardium is relaxed. The right ventricle receives blood from the venae cavae and coronary circulation, and pumps it via the pulmonary vasculature into the LV. Since PVR is a fraction of SVR, pulmonary arterial pressures are relatively low and the wall thickness of the right ventricle (RV) is much less than that of the LV. The RV thus resembles a passive conduit rather than a pump. Coronary perfusion to the RV occurs continuously during systole and diastole because of the low intraventricular and intramural pressures. In spite of the anatomical differences, the mechanical behaviour of the RV and LV is very similar.