Advanced Greenhouse Horticulture New Technologies and Cultivation Practices
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Organic Horticulture in India
horticulturae Communication Organic Horticulture in India Sisir Mitra 1,* and Hidangmayum Devi 2 1 Section Tropical and Subtropical Fruits, International Society for Horticultural Science, Faculty of Horticulture, Bidhan Chandra Krishi ViswaVidyalaya, Mohanpur, B-12/48, Kalyani, Nadia, West Bengal 741252, India 2 Indian Council of Agriculture Research complex for NEH Region, Tripura Centre, Lembucherra, West Tripura 799210, India; [email protected] * Correspondence: [email protected]; Tel.: +91-943-217-4249 Academic Editor: Douglas D. Archbold Received: 1 December 2015; Accepted: 27 September 2016; Published: 26 October 2016 Abstract: During the previous three decades, organic produce has attracted the attention of a growing health-conscious population across the globe. Both international and domestic communities are becoming aware of issues like agrochemical residues, produce quality, and food safety. Worldwide, over 37.5 million ha of land (0.87% of total agricultural land) is being managed organically by 1.9 million producers in 164 countries. In addition, there is another 31 million ha certified for wild harvest collection. Global sales of organic products have reached U.S. $75 billion, with the U.S. and Europe as the largest consumers. The concept of organic farming is not new to the Indian farming community. Several forms of organic farming are successfully practiced in diverse climates, particularly in rain-fed, tribal mountains, and hilly areas of the country. Many of the forest products of economic importance, such as herbs and medicinal plants are in this category by default. The report of the Task Force on Organic Farming appointed by the Government of India noted the vast areas of the country where limited amounts of synthetic chemicals are used, although they have low productivity, but also which could have unexploited potential for organic agriculture. -

2021 Catalog
2021 NEW PRODUCTS G-Power Flip and Punch Spin Bait Designed by Aaron Martens, Walleye anglers across the Midwest have become Gamakatsu has developed the dependent upon the spin style hooks for walleye rigs. new G-Power Heavy Cover Flip The Spin Bait hook can be rigged behind spinner & Punch Hook. A step up from blades, prop blades or used the G-Finesse Heavy Cover alone with just a simple Hook, for serious flipping and bead in front of them. It’s punching with heavy fluorocarbon and braid. The TGW (Tournament unique design incorporates Grade Wire) hook, paired with its welded eye, make this the strongest Gamakatsu swivels that is Heavy Cover hook in Gamakatsu’s G-Series lineup. Ideal for larger baits independent of the hook, giving the hook more freedom to spin while and weights, punching through grass mats and flipping into heavy reducing line twist. The Spin Bait hook features Nano Smooth Coat for timber. G-Power Flip and Punch ideally matches to all types of cover stealth presentations and unsurpassed hook penetration and the bait and able to withstand extreme conditions. Page 26 keeper barbs on the shank hold live and plastic baits on more securely. Page 48 G-Power Stinger Trailer Hook The new G-Power Stinger Trailer Hook Superline Offset Round Bend brilliance comes from Gamakatsu’s famous Gamakatsu’s Superline Offset Round B10S series of fly hooks and the expertise Bend is designed with a heavier of Professional Bass angler Aaron Martens. Superline wire best suited for heavy The Stinger Trailer has a strategically braided and fluorocarbon lines. -

Rolling Stone Magazine's Top 500 Songs
Rolling Stone Magazine's Top 500 Songs No. Interpret Title Year of release 1. Bob Dylan Like a Rolling Stone 1961 2. The Rolling Stones Satisfaction 1965 3. John Lennon Imagine 1971 4. Marvin Gaye What’s Going on 1971 5. Aretha Franklin Respect 1967 6. The Beach Boys Good Vibrations 1966 7. Chuck Berry Johnny B. Goode 1958 8. The Beatles Hey Jude 1968 9. Nirvana Smells Like Teen Spirit 1991 10. Ray Charles What'd I Say (part 1&2) 1959 11. The Who My Generation 1965 12. Sam Cooke A Change is Gonna Come 1964 13. The Beatles Yesterday 1965 14. Bob Dylan Blowin' in the Wind 1963 15. The Clash London Calling 1980 16. The Beatles I Want zo Hold Your Hand 1963 17. Jimmy Hendrix Purple Haze 1967 18. Chuck Berry Maybellene 1955 19. Elvis Presley Hound Dog 1956 20. The Beatles Let It Be 1970 21. Bruce Springsteen Born to Run 1975 22. The Ronettes Be My Baby 1963 23. The Beatles In my Life 1965 24. The Impressions People Get Ready 1965 25. The Beach Boys God Only Knows 1966 26. The Beatles A day in a life 1967 27. Derek and the Dominos Layla 1970 28. Otis Redding Sitting on the Dock of the Bay 1968 29. The Beatles Help 1965 30. Johnny Cash I Walk the Line 1956 31. Led Zeppelin Stairway to Heaven 1971 32. The Rolling Stones Sympathy for the Devil 1968 33. Tina Turner River Deep - Mountain High 1966 34. The Righteous Brothers You've Lost that Lovin' Feelin' 1964 35. -

Comprehensive Assessment of Organic Apple Production in Vermont: Experience from Two Orchard Systems, 2006-2013 Terence L
University of Vermont ScholarWorks @ UVM Graduate College Dissertations and Theses Dissertations and Theses 2015 Comprehensive Assessment of Organic Apple Production in Vermont: Experience from Two Orchard Systems, 2006-2013 Terence L. Bradshaw University of Vermont Follow this and additional works at: https://scholarworks.uvm.edu/graddis Part of the Agricultural Economics Commons, and the Horticulture Commons Recommended Citation Bradshaw, Terence L., "Comprehensive Assessment of Organic Apple Production in Vermont: Experience from Two Orchard Systems, 2006-2013" (2015). Graduate College Dissertations and Theses. 327. https://scholarworks.uvm.edu/graddis/327 This Dissertation is brought to you for free and open access by the Dissertations and Theses at ScholarWorks @ UVM. It has been accepted for inclusion in Graduate College Dissertations and Theses by an authorized administrator of ScholarWorks @ UVM. For more information, please contact [email protected]. COMPREHENSIVE ASSESSMENT OF ORGANIC APPLE PRODUCTION IN VERMONT: EXPERIENCE FROM TWO ORCHARD SYSTEMS, 2006-2013 A Dissertation Presented by Terence L. Bradshaw to The Faculty of the Graduate College of The University of Vermont In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Specializing in Plant and Soil Science May, 2015 Defense Date: March 25, 2014 Dissertation Examination Committee: Josef H. Gorres, Ph. D., Advisor Robert L. Parsons, Ph.D., Chairperson Lorraine P. Berkett, Ph.D. Sidney C. Bosworth, Ph.D. Cynthia J. Forehand, Ph.D., Dean of the Graduate College ABSTRACT Despite substantial consumer demand and willingness to pay premium prices for organically grown fruit, apple growers in Vermont and other New England states have been slow to adopt certified organic practices. -

Weight and Lifestyle Inventory (Wali)
WEIGHT AND LIFESTYLE INVENTORY (Bariatric Surgery Version) © 2015 Thomas A. Wadden, Ph.D. and Gary D. Foster, Ph.D. 1 The Weight and Lifestyle Inventory (WALI) is designed to obtain information about your weight and dieting histories, your eating and exercise habits, and your relationships with family and friends. Please complete the questionnaire carefully and make your best guess when unsure of the answer. You will have an opportunity to review your answers with a member of our professional staff. Please allow 30-60 minutes to complete this questionnaire. Your answers will help us better identify problem areas and plan your treatment accordingly. The information you provide will become part of your medical record at Penn Medicine and may be shared with members of our treatment team. Thank you for taking the time to complete this questionnaire. SECTION A: IDENTIFYING INFORMATION ______________________________________________________________________________ 1 Name _________________________ __________ _______lbs. ________ft. ______inches 2 Date of Birth 3 Age 4 Weight 5 Height ______________________________________________________________________________ 6 Address ____________________ ________________________ ______________________/_______ yrs. 7 Phone: Cell 8 Phone: Home 9 Occupation/# of yrs. at job __________________________ 10 Today’s Date 11 Highest year of school completed: (Check one.) □ 6 □ 7 □ 8 □ 9 □ 10 □ 11 □ 12 □ 13 □ 14 □ 15 □ 16 □ Masters □ Doctorate Middle School High School College 12 Race (Check all that apply): □ American Indian □ Asian □ African American/Black □ Pacific Islander □White □ Other: ______________ 13 Are you Latino, Hispanic, or of Spanish origin? □ Yes □ No SECTION B: WEIGHT HISTORY 1. At what age were you first overweight by 10 lbs. or more? _______ yrs. old 2. What has been your highest weight after age 21? _______ lbs. -

Song Lyrics of the 1950S
Song Lyrics of the 1950s 1951 C’mon a my house by Rosemary Clooney Because of you by Tony Bennett Come on-a my house my house, I’m gonna give Because of you you candy Because of you, Come on-a my house, my house, I’m gonna give a There's a song in my heart. you Apple a plum and apricot-a too eh Because of you, Come on-a my house, my house a come on My romance had its start. Come on-a my house, my house a come on Come on-a my house, my house I’m gonna give a Because of you, you The sun will shine. Figs and dates and grapes and cakes eh The moon and stars will say you're Come on-a my house, my house a come on mine, Come on-a my house, my house a come on Come on-a my house, my house, I’m gonna give Forever and never to part. you candy Come on-a my house, my house, I’m gonna give I only live for your love and your kiss. you everything It's paradise to be near you like this. Because of you, (instrumental interlude) My life is now worthwhile, And I can smile, Come on-a my house my house, I’m gonna give you Christmas tree Because of you. Come on-a my house, my house, I’m gonna give you Because of you, Marriage ring and a pomegranate too ah There's a song in my heart. -

Effect of Different Organic Wastes on Soil Pro- Perties and Plant Growth and Yield: a Review
ENVIRONMENTAL SCIENCES EFFECT OF DIFFERENT ORGANIC WASTES ON SOIL PRO- pertieS AND PLANT GROWTH AND YIELD: A REVIEW M.Z. Hossain1, P. von Fragstein und Niemsdorff2, J. Heß2 1Khulna University, Agrotechnology Discipline, Khulna, Bangladesh 2University of Kassel, Faculty of Organic Agricultural Sciences, Department of Organic Farming and Cropping System, Nordbahnhofstr. 1a, 37213 Witzenhausen, Germany The use of organic wastes in agriculture plays a great role in recycling essential plant nutrients, sustaining soil security as well as protecting the environment from unwanted hazards. This review article deals with the effect of different kinds of organic wastes on soil properties and plant growth and yield. Municipal solid waste is mainly used as a source of nitrogen and organic matter, improving soil properties and microbial activity that are closely related to soil fertility. Biowaste and food waste in- crease pH, nitrogen content, cation exchange capacity, water holding capacity, and microbial biomass in soil. Sewage sludge contains various amounts of organic matter and huge amounts of plant nutrients. Manure is a common waste which improves soil properties by adding nutrients and increases microbial and enzyme activity in soil. It also reduces toxicity of some heavy metals. These organic wastes have a great positive impact on soil physical, chemical, and biological properties as well as stimulate plant growth and thus increase the yield of crops. municipal solid waste, soil nutrients, sewage sludge, food waste, manure doi: 10.1515/sab-2017-0030 Received for publication on October 14, 2016 Accepted for publication on April 22, 2017 INTRODUCTION puropse to improve soil properties and increase crop yield is a good solution for minimizing these problems. -

Garden Organic (Formerly Known As HDRA - the Henry Doubleday Research Association) Is the UK’S Leading Organic Growing Charity
Composting for Gardeners Image credit: iStock/Allkindza Composting for Gardeners Garden Organic (formerly known as HDRA - the Henry Doubleday Research Association) is the UK’s leading organic growing charity. We have been at the forefront of the organic horticulture movement since 1958, with over 20,000 members across the UK and overseas. Dedicated to promoting organic gardening in homes, communities and schools, the charity encourages people to grow in the most sustainable way, and demonstrates the lasting benefi ts of organic growing to the health and wellbeing of individuals and the environment. For more information please visit www.gardenorganic.org.uk. Garden Organic is a registered charity in England and Wales (298104) and Scotland (SC046767). VAT no. 258 0869 69 Why make compost? Compost is nature’s black gold. It will help you grow healthy plants. And making it yourself reduces pollution, supports the natural environment, saves money and protects endangered natural habitats. How? By recycling your garden debris and kitchen scraps. It’s as simple as that. This book will tell you what to compost, how to make it, and how to use it. Compost makes your garden grow Compost, a rich soil-like material, works wonders in the growing area. It • lightens and breaks up heavy soils • bulks up light sandy soils and helps them hold more water • feeds plants and other vital soil organisms • helps control diseases Compost saves you money Home-made compost helps cut down on buying garden products. It acts as a • soil improver • fertiliser • mulch Compost helps reduce pollution Making compost contributes towards a cleaner environment. -

Before Using Scale Weight Measurement Only Personal Data
Congratulations on purchasing this Bluetooth® The scale will now switch to Age setting mode. After a few seconds, the LCD will show your body weight, body fat percentage, • Skin temperature can have an influence also. Measuring body fat in warm, humid Bone mass – what is it? Before Using Scale body water percentage, BMI, bone mass and muscle mass percentage for several weather when skin is moist will yield a different result than if skin is cold and dry. Bone is a living, growing tissue. During youth, your body makes new bone tissue connected Weight Watchers Scales by Conair™ Age will flash. Press the UP or DOWN button to choose the seconds, and then turn off automatically. • As with weight, when your goal is to change body composition, it is better to track faster than it breaks down older bone. In young adulthood, bone mass is at its peak; Precautions for Use Body Analysis Monitor! age (10 to 100). Pressing and holding the UP or DOWN button trends over time than to use individual daily results. after that, bone loss starts to outpace bone growth, and bone mass decreases. CAUTION! Use of this device by persons with any electrical implant such will advance numbers quickly. Press the SET button to confirm • Results may not be accurate for persons under the age of 16, or persons with an But it’s a long and very slow process that can be slowed down even more through It is designed to work with the free Weight Watchers Scales by as a heart pacemaker, or by pregnant women, is not recommended. -

Roadside Safety Design and Devices International Workshop
TRANSPORTATION RESEARCH Number E-C172 February 2013 Roadside Safety Design and Devices International Workshop July 17, 2012 Milan, Italy TRANSPORTATION RESEARCH BOARD 2013 EXECUTIVE COMMITTEE OFFICERS Chair: Deborah H. Butler, Executive Vice President, Planning, and CIO, Norfolk Southern Corporation, Norfolk, Virginia Vice Chair: Kirk T. Steudle, Director, Michigan Department of Transportation, Lansing Division Chair for NRC Oversight: Susan Hanson, Distinguished University Professor Emerita, School of Geography, Clark University, Worcester, Massachusetts Executive Director: Robert E. Skinner, Jr., Transportation Research Board TRANSPORTATION RESEARCH BOARD 2012–2013 TECHNICAL ACTIVITIES COUNCIL Chair: Katherine F. Turnbull, Executive Associate Director, Texas A&M Transportation Institute, Texas A&M University System, College Station Technical Activities Director: Mark R. Norman, Transportation Research Board Paul Carlson, Research Engineer, Texas A&M Transportation Institute, Texas A&M University System, College Station, Operations and Maintenance Group Chair Thomas J. Kazmierowski, Manager, Materials Engineering and Research Office, Ontario Ministry of Transportation, Toronto, Canada, Design and Construction Group Chair Ronald R. Knipling, Principal, safetyforthelonghaul.com, Arlington, Virginia, System Users Group Chair Mark S. Kross, Consultant, Jefferson City, Missouri, Planning and Environment Group Chair Joung Ho Lee, Associate Director for Finance and Business Development, American Association of State Highway and Transportation -

Innovative Strategies and Machines for Physical Weed Control in Organic and Integrated Vegetable Crops
211 A publication of CHEMICAL ENGINEERING TRANSACTIONS The Italian Association VOL. 44, 2015 of Chemical Engineering Online at www.aidic.it/cet Guest Editors: Riccardo Guidetti, Luigi Bodria, Stanley Best Copyright © 2015, AIDIC Servizi S.r.l., ISBN 978-88-95608-35-8; ISSN 2283-9216 DOI: 10.3303/CET1544036 Innovative Strategies and Machines for Physical Weed Control in Organic and Integrated Vegetable Crops Marco Fontanelli*a, Christian Frasconia, Luisa Martellonib, Michel Pirchiob, Michele a a Raffaelli , Andrea Peruzzi aDepartment of Agriculture Food and Environment, University of Pisa, Via del Borghetto 80, 56124, Pisa, Italy b Centro di Ricerche Agro-Ambientali “Enrico Avanzi”, Università di Pisa, Via vecchia di Marina 6, 56122, San PIero a Grado, Pisa, Italy [email protected] Weed control is one of the most serious problems in vegetable crops, limiting cultivated plants correct development, yields, product quality and farmers income. Therefore, the aim of this work was to set up and improve innovative strategies and machines for physical (mechanical and thermal) weed control in organic or “integrated” vegetables production in many important areas of Northern, Central and Southern Italy. Therefore, on-farm experiments were carried out since 1999 on fresh marketable spinach, processing and fresh market tomato, cauliflower, savoy cabbage, greenhouse cultivated leaf beet, garlic, chicory, fennel and carrot. These research activities started are still ongoing. The traditional farm weed management system was always compared to one or more innovative strategies that were defined according to the characteristics of the environment (i.e. soil type and conditions, water availability, etc.), typology of cultivation, crop rotation, expected technical and economical results. -
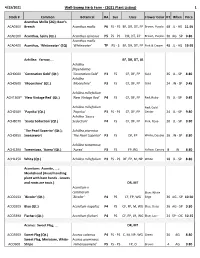
WSHF Catalog
4/23/2021 Well-Sweep Herb Farm - (2021 Plant Listing) 1 Stock # Common Botanical HA Sun Uses Flower ColorHT When Price Acanthus Mollis (2Q); Bear's ACA030X Breech Acanthus mollis P6 FS - PS BF, DR, DT, FP Brown, Purple- 48 JL - AG 11.95 ACA010X Acanthus, Spiny (Qt.) Acanthus spinosus P5 FS - PS DR, DT, FP Brown, Purple- 30 AG - SP 9.80 Acanthus mollis ACA040X Acanthus, `Whitewater' (2Q) `Whitewater' TP PS - S BF, DR, DT, FP Pink & Cream 48 JL - AG 19.95 Achillea: Yarrow, ... BF, DR, DT, LB Achillea filipendulina ACH000X `Coronation Gold' (Qt.) `Coronation Gold' P3 FS CF, DF, FP Gold 36 JL - SP 8.80 Achillea ACH050X `Moonshine' (Qt.) `Moonshine' P3 FS CF, DF, FP Gold 24 JN - SP 9.45 Achillea millefolium ACH130X* `New Vintage Red' (Qt.) `New Vintage Red' P4 FS CF, DF, FP Red, Ruby- 15 JL - SP 9.45 Achillea millefolium Red; Gold ACH250X `Paprika' (Qt.) `Paprika' P3 FS - PS CF, DF, FP Center 24 JL - SP 9.80 Achillea `Saucy ACH807X `Saucy Seduction' (Qt.) Seduction' P4 FS CF, DF, FP Pink, Rose- 20 JL - SP 9.80 `The Pearl Superior' (Qt.); Achillea ptarmica ACH095X Sneezewort `The Pearl Superior' P3 FS DF, FP White; Double 16 JN - SP 8.80 Achillea tomentosa ACH120X Tomentosa, `Aurea' (Qt.) `Aurea' P3 FS FP, RG Yellow, Canary- 8 JN 8.80 ACH125X White (Qt.) Achillea millefolium P3 FS - PS DF, FP, M, NP White 18 JL - SP 8.80 Aconitum: Aconite, ... ; Monkshood (Avoid handling plant with bare hands - Leaves and roots are toxic.) DR, MT Aconitum x cammarum Blue; White ACO015X `Bicolor' (Qt.) `Bicolor' P4 PS CF, FP, WG Edge 36 AG - SP 10.50 ACO020X Blue (Qt.) Aconitum napellus P4 PS CF, FP, M, WG Blue, Deep- 36 AG - SP 9.80 ACO339X Fischeri (Qt.) Aconitum fischeri P4 PS CF, FP, LB, WG Blue, Lav.- 24 SP - OC 10.15 Acorus: Sweet Flag, ..