E Coli Genetic Modification Expression Vector
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recombinant DNA and Elements Utilizing Recombinant DNA Such As Plasmids and Viral Vectors, and the Application of Recombinant DNA Techniques in Molecular Biology
Fact Sheet Describing Recombinant DNA and Elements Utilizing Recombinant DNA Such as Plasmids and Viral Vectors, and the Application of Recombinant DNA Techniques in Molecular Biology Compiled and/or written by Amy B. Vento and David R. Gillum Office of Environmental Health and Safety University of New Hampshire June 3, 2002 Introduction Recombinant DNA (rDNA) has various definitions, ranging from very simple to strangely complex. The following are three examples of how recombinant DNA is defined: 1. A DNA molecule containing DNA originating from two or more sources. 2. DNA that has been artificially created. It is DNA from two or more sources that is incorporated into a single recombinant molecule. 3. According to the NIH guidelines, recombinant DNA are molecules constructed outside of living cells by joining natural or synthetic DNA segments to DNA molecules that can replicate in a living cell, or molecules that result from their replication. Description of rDNA Recombinant DNA, also known as in vitro recombination, is a technique involved in creating and purifying desired genes. Molecular cloning (i.e. gene cloning) involves creating recombinant DNA and introducing it into a host cell to be replicated. One of the basic strategies of molecular cloning is to move desired genes from a large, complex genome to a small, simple one. The process of in vitro recombination makes it possible to cut different strands of DNA, in vitro (outside the cell), with a restriction enzyme and join the DNA molecules together via complementary base pairing. Techniques Some of the molecular biology techniques utilized during recombinant DNA include: 1. -

Gene Therapy Glossary of Terms
GENE THERAPY GLOSSARY OF TERMS A • Phase 3: A phase of research to describe clinical trials • Allele: one of two or more alternative forms of a gene that that gather more information about a drug’s safety and arise by mutation and are found at the same place on a effectiveness by studying different populations and chromosome. different dosages and by using the drug in combination • Adeno-Associated Virus: A single stranded DNA virus that has with other drugs. These studies typically involve more not been found to cause disease in humans. This type of virus participants.7 is the most frequently used in gene therapy.1 • Phase 4: A phase of research to describe clinical trials • Adenovirus: A member of a family of viruses that can cause occurring after FDA has approved a drug for marketing. infections in the respiratory tract, eye, and gastrointestinal They include post market requirement and commitment tract. studies that are required of or agreed to by the study • Adeno-Associated Virus Vector: Adeno viruses used as sponsor. These trials gather additional information about a vehicles for genes, whose core genetic material has been drug’s safety, efficacy, or optimal use.8 removed and replaced by the FVIII- or FIX-gene • Codon: a sequence of three nucleotides in DNA or RNA • Amino Acids: building block of a protein that gives instructions to add a specific amino acid to an • Antibody: a protein produced by immune cells called B-cells elongating protein in response to a foreign molecule; acts by binding to the • CRISPR: a family of DNA sequences that can be cleaved by molecule and often making it inactive or targeting it for specific enzymes, and therefore serve as a guide to cut out destruction and insert genes. -

Cloning of Gene Coding Glyceraldehyde-3-Phosphate Dehydrogenase Using Puc18 Vector
Available online a t www.pelagiaresearchlibrary.com Pelagia Research Library European Journal of Experimental Biology, 2015, 5(3):52-57 ISSN: 2248 –9215 CODEN (USA): EJEBAU Cloning of gene coding glyceraldehyde-3-phosphate dehydrogenase using puc18 vector Manoj Kumar Dooda, Akhilesh Kushwaha *, Aquib Hasan and Manish Kushwaha Institute of Transgene Life Sciences, Lucknow (U.P), India _____________________________________________________________________________________________ ABSTRACT The term recombinant DNA technology, DNA cloning, molecular cloning, or gene cloning all refers to the same process. Gene cloning is a set of experimental methods in molecular biology and useful in many areas of research and for biomedical applications. It is the production of exact copies (clones) of a particular gene or DNA sequence using genetic engineering techniques. cDNA is synthesized by using template RNA isolated from blood sample (human). GAPDH (Glyceraldehyde 3-phosphate dehydrogenase) is one of the most commonly used housekeeping genes used in comparisons of gene expression data. Amplify the gene (GAPDH) using primer forward and reverse with the sequence of 5’-TGATGACATCAAGAAGGTGGTGAA-3’ and 5’-TCCTTGGAGGCCATGTGGGCCAT- 3’.pUC18 high copy cloning vector for replication in E. coli, suitable for “blue-white screening” technique and cleaved with the help of SmaI restriction enzyme. Modern cloning vectors include selectable markers (most frequently antibiotic resistant marker) that allow only cells in which the vector but necessarily the insert has been transfected to grow. Additionally the cloning vectors may contain color selection markers which provide blue/white screening (i.e. alpha complementation) on X- Gal and IPTG containing medium. Keywords: RNA isolation; TRIzol method; Gene cloning; Blue/white screening; Agarose gel electrophoresis. -

Lecture 3 Types, Biology and Salient Features of Vectors in Recombinant
NPTEL – Bio Technology – Genetic Engineering & Applications MODULE 1- LECTURE 3 TYPES, BIOLOGY AND SALIENT FEATURES OF VECTORS IN RECOMBINANT DNA TECHNOLOGY – PLASMID 1-3.1 Introduction: DNA molecule used for carrying an exogenous DNA into a host organism and facilitates stable integration and replication inside the host system is termed as Vector. Molecular cloning involves series of sequential steps which includes restriction digestion of DNA fragments both target DNA and vector, ligation of the target DNA with the vector and introduction into a host organism for multiplication. Then the fragments resulted after digestion with restriction enzymes are ligated to other DNA molecules that serve as vectors. In general, vectors should have following characteristics: • Capable of replicating inside the host. • Have compatible restriction site for insertion of DNA molecule (insert). • Capable of autonomous replication inside the host (ori site). • Smaller in size and able to incorporate larger insert size. • Have a selectable marker for screening of recombinant organism. 1-3.2 Plasmids: • Plasmids are naturally occurring extra chromosomal double-stranded circular DNA molecules which can autonomously replicate inside bacterial cells. Plasmids range in size from about 1.0 kb to over 250 kb. • Plasmids encode only few proteins required for their own replication (replication proteins) and these proteins encoding genes are located very close to the ori. All the other proteins required for replication, e.g. DNA polymerases, DNA ligase, helicase, etc., are provided by the host cell.Thus, only a small region surrounding Joint initiative of IITs and IISc – Funded by MHRD Page 28 of 84 NPTEL – Bio Technology – Genetic Engineering & Applications the ori site is required for replication. -

Molecular Biology and Applied Genetics
MOLECULAR BIOLOGY AND APPLIED GENETICS FOR Medical Laboratory Technology Students Upgraded Lecture Note Series Mohammed Awole Adem Jimma University MOLECULAR BIOLOGY AND APPLIED GENETICS For Medical Laboratory Technician Students Lecture Note Series Mohammed Awole Adem Upgraded - 2006 In collaboration with The Carter Center (EPHTI) and The Federal Democratic Republic of Ethiopia Ministry of Education and Ministry of Health Jimma University PREFACE The problem faced today in the learning and teaching of Applied Genetics and Molecular Biology for laboratory technologists in universities, colleges andhealth institutions primarily from the unavailability of textbooks that focus on the needs of Ethiopian students. This lecture note has been prepared with the primary aim of alleviating the problems encountered in the teaching of Medical Applied Genetics and Molecular Biology course and in minimizing discrepancies prevailing among the different teaching and training health institutions. It can also be used in teaching any introductory course on medical Applied Genetics and Molecular Biology and as a reference material. This lecture note is specifically designed for medical laboratory technologists, and includes only those areas of molecular cell biology and Applied Genetics relevant to degree-level understanding of modern laboratory technology. Since genetics is prerequisite course to molecular biology, the lecture note starts with Genetics i followed by Molecular Biology. It provides students with molecular background to enable them to understand and critically analyze recent advances in laboratory sciences. Finally, it contains a glossary, which summarizes important terminologies used in the text. Each chapter begins by specific learning objectives and at the end of each chapter review questions are also included. -

The Effect of Increasing Plasmid Size on Transformation Efficiency in Escherichia Coli
Journal of Experimental Microbiology and Immunology (JEMI) Vol. 2:207-223 Copyright April 2002, M&I UBC The Effect of Increasing Plasmid Size on Transformation Efficiency in Escherichia coli VICKY CHAN, LISA F. DREOLINI, KERRY A. FLINTOFF, SONJA J. LLOYD, AND ANDREA A. MATTENLEY Department of Microbiology and Immunology, UBC Based on the observation that the transformation of Escherchia coli was more efficient with pUC19 than with the larger plasmid pBR322, we hypothesized that transformation frequency is somehow affected by size. To test this hypothesis, we attempted to insert a 1.7kb lambda NdeI fragment into pUC19 to generate a plasmid (pHEL) of the same size as pBR322. The two plasmids of equal size were then to be used to transform E. coli in order to compare transformation efficiencies. After two rounds of cloning, we were unable to generate pHEL. In lieu of using pHEL and pBR322, E. coli were transformed with previously prepared plasmids of varying sizes: pUC8 (2.6 kb), pUC8 0-690 (4.3 kb), and pUC8 0-690::pKT210 (16.1 kb). The results of these transformations indicate that increasing plasmid size correlates with a decrease in transformation efficiency. Transformation is an important technique in molecular cloning for transferring genetic material to bacteria. It can be done by either heat shock or electroporation. The former involves the preparation of competent cells, incubation of the cells with DNA at 0oC and the completion of DNA uptake by heat pulse. Competent cells are capable of taking up DNA. They can be prepared by cold treatment with calcium chloride. -

Regulation of Transgene Expression in Genetic Immunization
Brazilian Journal of Medical and Biological Research (1999) 32: 155-162 Regulation of transgene expression 155 ISSN 0100-879X Regulation of transgene expression in genetic immunization J.S. Harms1, 1Department of Animal Health and Biomedical Sciences, University of Wisconsin, S.C. Oliveira2 Madison, WI, USA and G.A. Splitter1 2Departamento de Bioquímica e Imunologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil Abstract Correspondence The use of mammalian gene expression vectors has become increas- Key words J.S. Harms ingly important for genetic immunization and gene therapy as well as · DNA vaccine AHABS basic research. Essential for the success of these vectors in genetic · Gene therapy 1655 Linden Drive · immunization is the proper choice of a promoter linked to the antigen Regulation Madison, WI 53706 · Transcription of interest. Many genetic immunization vectors use promoter elements USA · Transgene Fax: +1-608-262-7420 from pathogenic viruses including SV40 and CMV. Lymphokines E-mail: [email protected] produced by the immune response to proteins expressed by these vectors could inhibit further transcription initiation by viral promot- Research supported by the Robert ers. Our objective was to determine the effect of IFN-g on transgene Draper Technology Innovation Fund expression driven by viral SV40 or CMV promoter/enhancer and the (No. 135-0546) of the University mammalian promoter/enhancer for the major histocompatibility com- of Wisconsin, Madison. plex class I (MHC I) gene. We transfected the luciferase gene driven Presented at the International by these three promoters into 14 cell lines of many tissues and several Symposium “The Third Revolution species. -

RNA Viruses As Tools in Gene Therapy and Vaccine Development
G C A T T A C G G C A T genes Review RNA Viruses as Tools in Gene Therapy and Vaccine Development Kenneth Lundstrom PanTherapeutics, Rte de Lavaux 49, CH1095 Lutry, Switzerland; [email protected]; Tel.: +41-79-776-6351 Received: 31 January 2019; Accepted: 21 February 2019; Published: 1 March 2019 Abstract: RNA viruses have been subjected to substantial engineering efforts to support gene therapy applications and vaccine development. Typically, retroviruses, lentiviruses, alphaviruses, flaviviruses rhabdoviruses, measles viruses, Newcastle disease viruses, and picornaviruses have been employed as expression vectors for treatment of various diseases including different types of cancers, hemophilia, and infectious diseases. Moreover, vaccination with viral vectors has evaluated immunogenicity against infectious agents and protection against challenges with pathogenic organisms. Several preclinical studies in animal models have confirmed both immune responses and protection against lethal challenges. Similarly, administration of RNA viral vectors in animals implanted with tumor xenografts resulted in tumor regression and prolonged survival, and in some cases complete tumor clearance. Based on preclinical results, clinical trials have been conducted to establish the safety of RNA virus delivery. Moreover, stem cell-based lentiviral therapy provided life-long production of factor VIII potentially generating a cure for hemophilia A. Several clinical trials on cancer patients have generated anti-tumor activity, prolonged survival, and -

Clone the Unclonable – Vectors and Cells to Capture and Express Problematic Dnas
Clone the Unclonable – Vectors and Cells to Capture and Express Problematic DNAs Ron Godiska, Ph.D. May, 2016 Agenda Improve Cloning and Expression of Problematic DNA • What makes DNA difficult to clone or express? • Circular vectors for difficult DNAs • Linear vectors for “impossible” DNAs • Enzyme-free cloning and compatible protein expression vectors • E. coli strains for toxic recombinant proteins or unstable DNA What is Unclonable DNA? Characteristics of Difficult DNA • Toxic coding sequences • Promoters • A-T Rich DNA • Large fragments (>10 kb) • Trace amounts Which types of difficult DNA have you worked with in the lab? Common Vector Traits Cause Cloning Problems Vector driven transcription into the insert – Deleterious expression – Destabilize 2o structure Insert driven transcription out into the vector – False negatives (blue or lethal) – Transcriptional interference pUC19 – Replication interference High copy number – Increased effect of the above issues – Active origin – Many copies/cell Supercoiling – Deletion of secondary structures CloneSmart® Technology “Silencing” DNA Inserts to Improve Cloning • No Transcriptional Interference – Stably maintain “unclonable” DNA • No blue/white screening – No false positives/negatives • Minimal vector size • Variable copy number • Variety of drug selection markers • Very low empty-vector background U.S. Patent 6,709,861 pSMART Vectors Clone Toxic Inserts and Promoters Test DNA Fragments: • RNase coding sequence (350 bp; no promoter) • Phage Lambda PR Promoter (400 bp) pSMART Vectors cDNA Clones are More Stable Deletion Rates: <1% in pSMART HC Kan vs. 32% in pUC % Deleted Clones Insert Size (kb) • Teleost fish cDNA library • Clones were grown in liquid medium, diluted, and regrown • Each final culture was diluted, plated, and grown up into individual colonies • Plasmid DNA was isolated from colonies for each clone and analyzed - Oleksiak M.F., Crawford D.L., 2001. -

Molecular Cloning: a Laboratory Manual, 4Th Edition
This is a free sample of content from Molecular Cloning: A Laboratory Manual, 4th edition. Click here for more information or to buy the book. VOLUME 1 Molecular Cloning A LABORATORY MANUAL FOURTH EDITION © 2012 by Cold Spring Harbor Laboratory Press This is a free sample of content from Molecular Cloning: A Laboratory Manual, 4th edition. Click here for more information or to buy the book. OTHER TITLES FROM CSHL PRESS LABORATORY MANUALS Antibodies: A Laboratory Manual Imaging: A Laboratory Manual Live Cell Imaging: A Laboratory Manual, 2nd Edition Manipulating the Mouse Embryo: A Laboratory Manual, 3rd Edition RNA: A Laboratory Manual HANDBOOKS Lab Math: A Handbook of Measurements, Calculations, and Other Quantitative Skills for Use at the Bench Lab Ref, Volume 1: A Handbook of Recipes, Reagents, and Other Reference Tools for Use at the Bench Lab Ref, Volume 2: A Handbook of Recipes, Reagents, and Other Reference Tools for Use at the Bench Statistics at the Bench: A Step-by-Step Handbook for Biologists WEBSITES Molecular Cloning, A Laboratory Manual, 4th Edition, www.molecularcloning.org Cold Spring Harbor Protocols, www.cshprotocols.org © 2012 by Cold Spring Harbor Laboratory Press This is a free sample of content from Molecular Cloning: A Laboratory Manual, 4th edition. Click here for more information or to buy the book. VOLUME 1 Molecular Cloning A LABORATORY MANUAL FOURTH EDITION Michael R. Green Howard Hughes Medical Institute Programs in Gene Function and Expression and in Molecular Medicine University of Massachusetts Medical School Joseph Sambrook Peter MacCallum Cancer Centre and the Peter MacCallum Department of Oncology The University of Melbourne, Australia COLD SPRING HARBOR LABORATORY PRESS Cold Spring Harbor, New York † www.cshlpress.org © 2012 by Cold Spring Harbor Laboratory Press This is a free sample of content from Molecular Cloning: A Laboratory Manual, 4th edition. -
Important Vector Factors for Gene Expression Technical Reference Guide
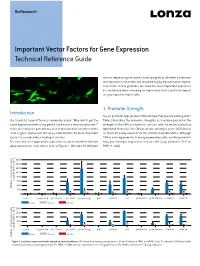
BioResearch Important Vector Factors for Gene Expression Technical Reference Guide vectors expressing the same luciferase gene in different backbones and expression cassettes and obtained highly discriminative expres- sion levels. In this guideline, we show the most important aspects to be considered when choosing an expression vector and their impact on your experimental results. 1. Promoter Strength Introduction Is your promoter appropriate for the cell type that you are working with? Our Scientific Support Team is commonly asked: “Why don’t I get the Table 1 describes the promoter strengths as a relative percent of the same expression level of my gene if I use various vector backbones?” strength of the CMV promoter for various cells for which Lonza has There are many components to a vector that can have an effect on the Optimized Protocols. The CMV promoter activity is set to 100% based level of gene expression. Below you will find the 10 most important on their CAT assay values from the referenced publications. Although factors to consider when looking at vectors. CMV is a strong promoter in many mammalian cells, another promoter The selection of an appropriate expression vector is crucial for efficient may give stronger expression in your cells (e.g., promoter SV40 in gene expression. Just take a look at Figure 1. We tried 10 different BHK-21 cells). n 1600 1400 1200 1000 800 [% of pGL3-CMV at 24 h] 24 at pGL3-CMV [% of 600 400 Relative luciferase-expressio Relative 200 0 Control Program Only pGL3-Control pGL3-CMV pmax Cloning™ pSG5 pcDNA3.1 pFP pIRES-MCS B pcDNA4 HisMax pCMVbeta pCMV-TNT 4 hours 24 hours 48 hours n 2000 1500 1000 500 0 Control Program Only pGL3-Control pGL3-CMV pmax Cloning™ pSG5 pcDNA3.1 pFP pIRES-MCS B pcDNA4 HisMax pCMVbeta pCMV-TNT [% of pGL3-CMV at 24 h] 24 at pGL3-CMV [% of 4 hours 24 hours 48 hours Relative luciferase-expressio Relative Figure 1: Luciferase expression levels depend on vector backbones. -

Cloning Technologies for Protein Expression and Purification
Cloning technologies for protein expression and purification James L Hartley Detailed knowledge of the biochemistry and structure of expedite the manipulations that are often required to individual proteins is fundamental to biomedical research. To obtain pure, active recombinant proteins. further our understanding, however, proteins need to be purified in sufficient quantities, usually from recombinant sources. Cloning for protein expression and Although the sequences of genomes are now produced in purification automated factories purified proteins are not, because their The required amount, purity, homogeneity, and activity behavior is much more variable. The construction of plasmids of the protein of interest usually determine the choice of and viruses to overexpress proteins for their purification is often expression context, vector and host. Usually the first tedious. Alternatives to traditional methods that are faster, easier consideration is what host cell to use. Expression in and more flexible are needed and are becoming available. Escherichia coli is fast, inexpensive and scaleable, and minimal post-translational modifications make proteins Addresses Protein Expression Laboratory, Research Technology Program, purified from E. coli relatively homogeneous and highly SAIC-Frederick, Inc., NCI-Frederick, Frederick, Maryland 21702, USA desirable for structural studies. However, in our experi- ence most eukaryotic proteins larger than 30 kDa are Corresponding author: Hartley, James L ([email protected]) unlikely to be properly folded when expressed in E. coli (JL Hartley et al., unpublished). Expression in mamma- Current Opinion in Biotechnology 2006, 17:359–366 lian cells is best for activity and native structure (includ- ing post-translational modifications), but yields are much This review comes from a themed issue on lower and costs are high.