Frequency‐Specific Neuromodulation of Local and Distant Connectivity In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modulation of Interhemispheric Inhibition Between Primary Motor Cortices Induced by Manual Motor Imitation: a Transcranial Magnetic Stimulation Study
brain sciences Article Modulation of Interhemispheric Inhibition between Primary Motor Cortices Induced by Manual Motor Imitation: A Transcranial Magnetic Stimulation Study Dongting Tian 1,*, Shin-ichi Izumi 1,2 and Eizaburo Suzuki 1,3 1 Department of Physical Medicine and Rehabilitation, Tohoku University Graduate School of Medicine, Sendai 980-8575, Japan; [email protected] (S.-i.I.); [email protected] (E.S.) 2 Department of Physical Medicine and Rehabilitation, Tohoku University Graduate School of Biomedical Engineering, Sendai 980-8575, Japan 3 Department of Physical Therapy, Yamagata Prefectural University of Health Sciences, 260 Kamiyanagi, Yamagata 990-2212, Japan * Correspondence: [email protected] Abstract: Imitation has been proven effective in motor development and neurorehabilitation. How- ever, the relationship between imitation and interhemispheric inhibition (IHI) remains unclear. Transcranial magnetic stimulation (TMS) can be used to investigate IHI. In this study, the modifica- tion effects of IHI resulting from mirror neuron system (MNS) activation during different imitations are addressed. We measured IHI between homologous primary motor cortex (M1) by analyzing the ipsilateral silent period (iSP) evoked by single-pulse focal TMS during imitation and analyzed the respective IHI modulation during and after different patterns of imitation. Our main results showed that throughout anatomical imitation, significant time-course changes of iSP duration through the Citation: Tian, D.; Izumi, S.-i.; experiment were observed in both directions. iSP duration declined from the pre-imitation time Suzuki, E. Modulation of Interhemispheric Inhibition between point to the post-imitation time point and did not return to baseline after 30 min rest. -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

Systematic Examination of Low-Intensity Ultrasound Parameters
TOOLS AND RESOURCES Systematic examination of low-intensity ultrasound parameters on human motor cortex excitability and behavior Anton Fomenko1†*, Kai-Hsiang Stanley Chen1,2†, Jean-Franc¸ois Nankoo1, James Saravanamuttu1, Yanqiu Wang1, Mazen El-Baba1, Xue Xia3, Shakthi Sanjana Seerala4, Kullervo Hynynen4, Andres M Lozano1,5*, Robert Chen1,3* 1Krembil Research Institute, University Health Network, Toronto, Canada; 2Department of Neurology, National Taiwan University Hospital Hsin-Chu Branch, Hsin-Chu, Taiwan; 3Division of Neurology, Department of Medicine, University of Toronto, Toronto, Canada; 4Sunnybrook Research Institute, Toronto, Canada; 5Division of Neurosurgery, Department of Surgery, Toronto Western Hospital, University of Toronto, Toronto, Canada Abstract Low-intensity transcranial ultrasound (TUS) can non-invasively modulate human neural activity. We investigated how different fundamental sonication parameters influence the effects of TUS on the motor cortex (M1) of 16 healthy subjects by probing cortico-cortical excitability and behavior. A low-intensity 500 kHz TUS transducer was coupled to a transcranial magnetic stimulation (TMS) coil. TMS was delivered 10 ms before the end of TUS to the left M1 hotspot of the first dorsal interosseous muscle. Varying acoustic parameters (pulse repetition frequency, duty *For correspondence: cycle, and sonication duration) on motor-evoked potential amplitude were examined. Paired-pulse [email protected] measures of cortical inhibition and facilitation, and performance on a visuomotor task was also (AF); assessed. TUS safely suppressed TMS-elicited motor cortical activity, with longer sonication [email protected] (AML); durations and shorter duty cycles when delivered in a blocked paradigm. TUS increased GABA - [email protected] (RC) A mediated short-interval intracortical inhibition and decreased reaction time on visuomotor task but † These authors contributed not when controlled with TUS at near-somatosensory threshold intensity. -
White Matter Tracts - Brain A143 (1)
WHITE MATTER TRACTS - BRAIN A143 (1) White Matter Tracts Last updated: August 8, 2020 CORTICOSPINAL TRACT .......................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 FUNCTION ............................................................................................................................................. 1 UNCINATE FASCICULUS ........................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 DTI PROTOCOL ...................................................................................................................................... 4 FUNCTION .............................................................................................................................................. 4 DEVELOPMENT ....................................................................................................................................... 4 CLINICAL SIGNIFICANCE ........................................................................................................................ 4 ARTICLES .............................................................................................................................................. -

Development and Prospect of Neuromodulation Technology
Development and Prospect of Neuromodulation Technology Hsin-Yi (Happy) Lai, PI Professor 赖欣怡, 教授 Why Study the Brain? SpiNNaker Chip 80-90 billion Neurons Robot Brain Disease Understanding Human Mind and Brain Function Neuroscience-inspired Artificial Intelligence New Diagnosis and Treatments for Brain Disease Brain Machine Interface Brain Stimulation chiefscientist.gov.au; www.swisswuff.ch; queuesquared.com; cannabisoilresearch.com; Johns Hopkins Applied Physics Lab Sensations, Memory, Emotion… Vision http://faculty.pasadena.edu/ How to Study the Brain? Brain Machine Interface Recording Neuromodulation Technology Brain Research Technology Medical Science Diagnosis and Therapy Neuromodulation Technology Electrical (DBS) Chemical Thermal Cryogenic Optical Magnetic (TMS) Mechanical (FUS) Invasive Neuromodulation Optical stimulation Microinjection Deep brain stimulation (DBS) http://www.tritechresearch.com/IMS-3.html Reversible cooling • targeting accuracy • brain tissue damage, adverse side effects http:// www.the-scientist.com • Any new applications for other brain diseases and disorder? Ex: Epilepsy, Dysmyotonia, Obsessive, Depression, etc. Sumner et al., Nat Neuroscience, 2008 https://www.youtube.com/watch?v=7Mmsah0v9Qc Noninvasive Neuromodulation Transcranial magnetic Transcranial direct stimulation (TMS) current stimulation (tDCS) http://www.drchugh.com/rtms.html 1–2 mA http://davidileitman.com/time-causality-and-perception-tcp/ neurology and mental health cognitive functions Limitation of spatial resolution !!! Noninvasive Neuromodulation Temporal interference Focused ultrasound (FUS) stimulation (TIS) Using TMS or TIS to directly stimulate deep brain structures requires stronger stimulation of overlying (eg, cortical) areas, which may result in unanticipated adverse effects and encroach on safety guidelines. Alan Urban, et al, 2015 Nature Meth Dmochowski et al., 2017 Cell • high spatial selectivity TIS in humans is a challenging • penetration depth and promising technique. -

Memory Loss from a Subcortical White Matter Infarct
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.51.6.866 on 1 June 1988. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:866-869 Short report Memory loss from a subcortical white matter infarct CAROL A KOOISTRA, KENNETH M HEILMAN From the Department ofNeurology, College ofMedicine, University ofFlorida, and the Research Service, Veterans Administration Medical Center, Gainesville, FL, USA SUMMARY Clinical disorders of memory are believed to occur from the dysfunction of either the mesial temporal lobe, the mesial thalamus, or the basal forebrain. Fibre tract damage at the level of the fornix has only inconsistently produced amnesia. A patient is reported who suffered a cerebro- vascular accident involving the posterior limb of the left internal capsule that resulted in a persistent and severe disorder of verbal memory. The inferior extent of the lesion effectively disconnected the mesial thalamus from the amygdala and the frontal cortex by disrupting the ventral amygdalofugal and thalamic-frontal pathways as they course through the diencephalon. This case demonstrates that an isolated lesion may cause memory loss without involvement of traditional structures associated with memory and may explain memory disturbances in other white matter disease such as multiple sclerosis and lacunar state. Protected by copyright. Memory loss is currently believed to reflect grey day of his illness the patient was transferred to Shands matter damage of either the mesial temporal lobe,' -4 Teaching Hospital at the University of Florida for further the mesial or the basal forebrain.'0 l evaluation. thalamus,5-9 Examination at that time showed the patient to be awake, Cerebrovascular accidents resulting in memory dys- alert, attentive and fully oriented. -

White Matter Dissection and Structural Connectivity of the Human Vertical
www.nature.com/scientificreports OPEN White matter dissection and structural connectivity of the human vertical occipital fasciculus to link vision-associated brain cortex Tatsuya Jitsuishi1, Seiichiro Hirono2, Tatsuya Yamamoto1,3, Keiko Kitajo1, Yasuo Iwadate2 & Atsushi Yamaguchi1* The vertical occipital fasciculus (VOF) is an association fber tract coursing vertically at the posterolateral corner of the brain. It is re-evaluated as a major fber tract to link the dorsal and ventral visual stream. Although previous tractography studies showed the VOF’s cortical projections fall in the dorsal and ventral visual areas, the post-mortem dissection study for the validation remains limited. First, to validate the previous tractography data, we here performed the white matter dissection in post-mortem brains and demonstrated the VOF’s fber bundles coursing between the V3A/B areas and the posterior fusiform gyrus. Secondly, we analyzed the VOF’s structural connectivity with difusion tractography to link vision-associated cortical areas of the HCP MMP1.0 atlas, an updated map of the human cerebral cortex. Based on the criteria the VOF courses laterally to the inferior longitudinal fasciculus (ILF) and craniocaudally at the posterolateral corner of the brain, we reconstructed the VOF’s fber tracts and found the widespread projections to the visual cortex. These fndings could suggest a crucial role of VOF in integrating visual information to link the broad visual cortex as well as in connecting the dual visual stream. Te VOF is the fber tract that courses vertically at the posterolateral corner of the brain. Te VOF was histori- cally described in monkey by Wernicke1 and then in human by Obersteiner2. -
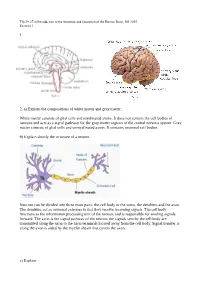
1. 2. A) Explain the Compositions of White Matter and Gray
Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 1. 2. a) Explain the compositions of white matter and gray matter. White matter consists of glial cells and myelinated axons. It does not contain the cell bodies of neurons and acts as a signal pathway for the gray matter regions of the central nervous system. Gray matter consists of glial cells and unmyelinated axons. It contains neuronal cell bodies. b) Explain shortly the structure of a neuron. Neurons can be divided into three main parts: the cell body or the soma, the dendrites and the axon. The dendrites act as neuronal antennas in that they receive incoming signals. The cell body functions as the information processing unit of the neuron, and is responsible for sending signals forward. The axon is the signal pathway of the neuron; the signals sent by the cell body are transmitted along the axon to the axon terminals located away from the cell body. Signal transfer is along the axon is aided by the myelin sheath that covers the axon. c) Explain: Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 i) myelin Membrane that wraps around axons. Formed from glial support cells: oligodendrogolia in the central nervous system and Schwann cells in the peripheral nervous system. Myelin facilitates signal transfer along axons by allowing action potentials to skip between the nodes of Ranvier (saltatory conduction). ii) receptor Molecule specialized in receiving a chemical signal by binding a specific neurotransmitter. -

Challenges in Neuromodulation Therapy
Challenges in Neuromodulation Therapy Milton M. Morris, PhD, MBA Principal MEH BioMedical, LLC February 25, 2015 Neuromodulation offers multiple indication therapy EMERGING Deep Brain Stim: Obesity, Stroke Recovery, Depression FDA APPROVED Cortical Stim: Epilepsy Deep Brain Stim: Peripheral Nerve Stim: Migraines, Extremity Pain Parkinson’s Disease, Dystonia, Essential Tremor, Obsessive Compulsive Disorder Carotid Artery, Sinus Stim: Hypertension Vagus Nerve Stimulation: Hypoglossal & Phrenic Nerve Stim: Sleep Apnea Depression, Epilepsy Spinal Cord: Spinal Cord Stim: Angina Pain Gastric Stim: Obesity Sacral Nerve Stim: Sacral & Pudendal Nerve Stim: Urinary Incontinence, Interstitial Cystitis, Sexual Function, Pelvic Pain Fecal Incontinence Percutaneous Tibial Nerve Stim: FUTURE Urinary Incontinence Deep Brain Stim: Alzheimer’s, Anxiety, Bulimia, Tinnitus, Traumatic Brain Injury, Tourette’s, Sleep Disorders, Autism, Bipolar Vagus Nerve Stim: Alzheimer’s, Anxiety, Obesity, Bulimia, Tinnitus, Obsessive Compulsive Disorder, Heart Failure Spinal Cord Stim: Asthma Gastric Stim: Bulimia, Interstitial Cystitis Status of Neuromodulation Therapy(ies) • FDA: Approved Epilepsy by the numbers • CMS: Favorable Coverage Recommendation Epilepsy 9 <3% chance of seizure th 4 most common MILLION freedom after 2 AED neurological disease after People living with epilepsy in migraine, stroke and failures United States, Europe, Alzheimer's disease and Japan Direct and indirect costs 400,000 of 25-40X People indicated for Mortality rate vs. VNS Therapy® $13.5B general population in US per year in US alone Source: CDC, WHO, IOC report on Epilepsy Status of Neuromodulation Therapy(ies) • FDA: Approved (VNS) Depression by the numbers • CMS: Non-Favorable Coverage Recommendation Depression 18 350 Major depressive MILLION Million disorder (MDD) is the People affected at any People worldwide second leading cause of one time in United affected by depressive disability worldwide* States illness 4 Direct and indirect costs of >39,000 Million > $43B Suicide deaths per year approx. -

The Nomenclature of Human White Matter Association Pathways: Proposal for a Systematic Taxonomic Anatomical Classification
The Nomenclature of Human White Matter Association Pathways: Proposal for a Systematic Taxonomic Anatomical Classification Emmanuel Mandonnet, Silvio Sarubbo, Laurent Petit To cite this version: Emmanuel Mandonnet, Silvio Sarubbo, Laurent Petit. The Nomenclature of Human White Matter Association Pathways: Proposal for a Systematic Taxonomic Anatomical Classification. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.94. 10.3389/fnana.2018.00094. hal-01929504 HAL Id: hal-01929504 https://hal.archives-ouvertes.fr/hal-01929504 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 06 November 2018 doi: 10.3389/fnana.2018.00094 The Nomenclature of Human White Matter Association Pathways: Proposal for a Systematic Taxonomic Anatomical Classification Emmanuel Mandonnet 1* †, Silvio Sarubbo 2† and Laurent Petit 3* 1Department of Neurosurgery, Lariboisière Hospital, Paris, France, 2Division of Neurosurgery, Structural and Functional Connectivity Lab, Azienda Provinciale per i Servizi Sanitari (APSS), Trento, Italy, 3Groupe d’Imagerie Neurofonctionnelle, Institut des Maladies Neurodégénératives—UMR 5293, CNRS, CEA University of Bordeaux, Bordeaux, France The heterogeneity and complexity of white matter (WM) pathways of the human brain were discretely described by pioneers such as Willis, Stenon, Malpighi, Vieussens and Vicq d’Azyr up to the beginning of the 19th century. -

10 Things to Know About Neuromodulation. Minimally Invasive Procedures to Reduce Or Alleviate Pain
NORTH AMERICAN NEUROMODULATION SOCIETY 4700 W. Lake Avenue Glenview, IL 60025 www.neuromodulation.org Rubenstein Public Relations Contact: Eve McGrath Tel: 212-843-8490 Email: [email protected] FOR IMMEDIATE RELEASE 10 Things to Know About Neuromodulation Minimally Invasive Procedures to Reduce or Alleviate Pain NEW YORK – February 24, 2010 – Robert Foreman, Ph.D., president of the North American Neuromodulation Society (NANS), stated, “Neuromodulation is among the most rapidly growing fields in medicine today. It can help to relieve chronic back pain, pain from cancer and other nerve injuries, pain from Complex Regional Pain Syndrome (CRPS) and Reflex Sympathetic Dystrophy (RSD) greatly improving the quality of life for patients.” Neuromodulation encompasses the application of targeted electrical, chemical and biological technologies to the nervous system in order to improve function and quality of life. The appropriate therapy (low level electrical pulses or micro-doses of medicine) are targeted to nerves along the spinal cord to block pain signals to the brain According to Joshua Prager, MD, MS, former president of NANS, “Neuromodulation can give people their lives back. Patients have gone from wheelchairs back to the tennis court, back to the sidelines of their children’s soccer games, back to their jobs. There are few treatments that can improve the activity level and the psychological outlook of a patient in pain like neuromodulation techniques.” NANS has compiled ten things everyone should know about neuromodulation: 1. Neuromodulation alleviates or lessens pain without putting patients into a “drug fog.” By relieving pain with neuro-stimulation or a drug-delivery system, that provides micro- doses of medicine, the patient can avoid some side effects, including excessive sedation or clouding of thoughts. -

Neuroanatomy
Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Neuroanatomy W. Jeffrey Wilson Fall 2012 \Without education we are in a horrible and deadly danger of taking educated people seriously." { Gilbert Keith Chesterton [LATEX in use { a Microsoft- & PowerPoint-free presentation] Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Blood-Brain Barrier Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Peripheral Nervous System • Somatic N.S.: skeletal muscles, skin, joints • Autonomic N.S.: internal organs, glands • Sympathetic N.S.: rapid expenditure of energy • Parasympathetic N.S.: restoration of energy Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Spinal Cord Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain | Ventricles Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain Midline Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain Midline Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Hindbrain Myelencephalon & Metencephalon Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Reticular