Red Crested Cardinal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CSE Big Brochure
PRODUCT CATALOG ULTIMATE OPTIQUE GLASS Cardinal is pleased to offer this industry-exclusive product combining cast and clear glass in the same panel. See page 14 for more details. www.cardinalshower.com LOUISVILLE MANUFACTURING FACILITY CARDINAL SHOWER ENCLOSURES Cardinal Shower Enclosures is a full service domestic manufacturer of shower enclosures, offering a wide selection of models, finishes and glass options, from cast to patterned glass. Our enclosures are engineered to the highest possible standards for maximum reliability and carry a lifetime guarantee against defects in craftsmanship and materials on extruded aluminum parts. With our network of trucks and locations across the U.S. we can reach 80% of the population with twice-a-week deliveries. From our in-house 80,000 square foot tempering facility, we can be your single source for enclosures and Venetian Cast Glass. While the industry standard is a 20% reject rate for tempered panels, we’re running at a less than 2% reject rate, virtually eliminating the need for return trips to the job. Our large 280,000 square foot manufacturing facility means we can provide hundreds of enclosures at a time for your large projects. We also produce all of our stunning cast glass in-house, ensuring the best quality for your enclosures and architectural glass applications. 2 SLIDING DOOR ENCLOSURES OPTIONS SPECIFICATIONS Cardinal Builder Series 4 Cast Glass Patterns 15 Cardinal Shower Enclosures, manufactured by Hoskin & Craftsman Builder Series 4 Heavy Glass Patterns 16 Muir, Inc., are fabricated with extruded 5000 and 6000 Craftsman Series 5 Thin Glass Patterns 17 series billet. All exposed surfaces are polished before anodizing in the Cardinal line. -
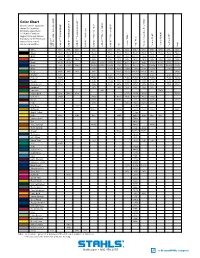
Color Chart ® ® ® ® Closest Pantone® Equivalent Shown
™ ™ II ® Color Chart ® ® ® ® Closest Pantone® equivalent shown. Due to printing limitations, colors shown 5807 Reflective ® ® ™ ® ® and Pantone numbers ® ™ suggested may vary from ac- ECONOPRINT GORILLA GRIP Fashion-REFLECT Reflective Thermo-FILM Thermo-FLOCK Thermo-GRIP ® ® ® ® ® ® ® tual colors. For the truest color ® representation, request Scotchlite our material swatches. ™ CAD-CUT 3M CAD-CUT CAD-CUT CAD-CUT CAD-CUT CAD-CUT CAD-CUT Felt Perma-TWILL Poly-TWILL Thermo-FILM Thermo-FLOCK Thermo-GRIP Vinyl Pressure Sensitive Poly-TWILL Sensitive Pressure CAD-CUT White White White White White White White White White* White White White White White Black Black Black Black Black Black Black Black Black* Black Black Black Black Black Gold 1235C 136C 137C 137C 123U 715C 1375C* 715C 137C 137C 116U Red 200C 200C 703C 186C 186C 201C 201C 201C* 201C 186C 186C 186C 200C Royal 295M 294M 7686C 2747C 7686C 280C 294C 294C* 294C 7686C 2758C 7686C 654C Navy 296C 2965C 7546C 5395M 5255C 5395M 276C 532C 532C* 532C 5395M 5255C 5395M 5395C Cool Gray Warm Gray Gray 7U 7539C 7539C 415U 7538C 7538C* 7538C 7539C 7539C 2C Kelly 3415C 341C 340C 349C 7733C 7733C 7733C* 7733C 349C 3415C Orange 179C 1595U 172C 172C 7597C 7597C 7597C* 7597C 172C 172C 173C Maroon 7645C 7645C 7645C Black 5C 7645C 7645C* 7645C 7645C 7645C 7449C Purple 2766C 7671C 7671C 669C 7680C 7680C* 7680C 7671C 7671C 2758U Dark Green 553C 553C 553C 447C 567C 567C* 567C 553C 553C 553C Cardinal 201C 188C 195C 195C* 195C 201C Emerald 348 7727C Vegas Gold 616C 7502U 872C 4515C 4515C 4515C 7553U Columbia 7682C 7682C 7459U 7462U 7462U* 7462U 7682C Brown Black 4C 4675C 412C 412C Black 4C 412U Pink 203C 5025C 5025C 5025C 203C Mid Blue 2747U 2945U Old Gold 1395C 7511C 7557C 7557C 1395C 126C Bright Yellow P 4-8C Maize 109C 130C 115U 7408C 7406C* 7406C 115U 137C Canyon Gold 7569C Tan 465U Texas Orange 7586C 7586C 7586C Tenn. -

CARDINAL FLOWER Lobelia Cardinalis
texas parks and wildlife CARDINAL FLOWER Lobelia cardinalis ©Jim Whitcomb Cardinal flower has vivid scarlet flowers that seem to be particularly attractive to hummingbirds. This plant blooms during late summer and early fall when hummingbirds are passing through on their southerly migration. Hummingbirds are attracted to plants with red tubular flowers. Range Plants CARDINAL FLOWER Lobelia cardinalis Appearance Life Cycle Height: 6 inches to 6 feet Plant type: Perennial Flower size: Individual flowers are 2 inches long on 8 inch spikes Bloom time: May-October Flower color: Intense red Method of reproduction: Layering. Bend a Cardinal flowers produce one to several stalks stem over and partially bury it. It will that are topped by spikes of deep velvet red produce new plants from the leaf nodes. flowers. They form basal rosettes in the winter. Planting time: Young plants should be transplanted during early spring or late fall. Planting Information Soil: Moist sand, loam, clay or limestone, poor Legend Has It ... drainage is okay Sunlight: Prefers partial shade The scarlet-red flower was named for Spacing: 1 foot apart the red robes worn by cardinals in the Lifespan: Short lived perennials whose size Catholic Church. Although native to varies according to environmental conditions. North America, it's been cultivated in Europe since the 1600s for its lovely flower. One legend claims that touching the root of this plant will bring love to Now You Know! the lives of elderly women! = Cardinal flowers grow tallest and flower best in wet, partially sunny areas. = Cardinal flowers time their blooms with the Habitat fall migration of hummingbirds, their chief pollinator. -

Pac-Clad® Color Chart
pa c - c l a d ® c O l O R c H a RT Cardinal Red Colonial Red Burgundy Terra Cotta Sierra Tan Mansard Brown Stone White Granite Sandstone Almond Medium Bronze Dark Bronze Slate Gray Bone White Musket Gray Charcoal Midnight Bronze Matte Black Cityscape Interstate Blue Hemlock Green Arcadia Green Patina Green Hunter Green Military Blue Award Blue Teal Hartford Green Forest Green Evergreen Denotes PAC-CLAD Metallic Colors Denotes Energy Star® Colors Denotes PAC-CLAD Cool Colors Kynar 500® or Hylar 5000® pre-finished galvanized steel and aluminum for roofing, curtainwall and storefront applications. Berkshire Blue Slate Blue PAC-CLAD Metallic Colors Zinc Silver Copper Penny Aged Copper Champagne Weathered Zinc PETERSEN ALUMINUM CORPORATION HQ: 1005 Tonne Road 9060 Junction Drive 10551 PAC Road 350 73rd Ave., NE, Ste 1 102 Northpoint Pkwy Ext, Bldg 1, Ste 100 Elk Grove Village, IL 60007 Annapolis Junction, MD 20701 Tyler, TX 75707 Fridley, MN 55432 Acworth, GA 30102 P: 800-PAC-CLAD P: 800-344-1400 P: 800-441-8661 P: 877-571-2025 P: 800-272-4482 F: 800-722-7150 F: 301-953-7627 F: 903-581-8592 F: 866-901-2935 F: 770-420-2533 pa c - c l a d ® c O l O R aVa I l a BI l ITY PAC-CLAD 3 year STeeL ALuMiNuM eNeRGy STANDARD RefLectiviTy EmissiviTy SRi 24ga. 22ga. .032 .040 .050 .063 ® COLORS exposuRe STAR Almond 0.56 0.83 0.27 64 √ √ √ √ √ • Arcadia Green 0.33 0.84 0.32 33 √ √ • Bone White 0.71 0.85 0.71 86 √ √ √ √ √ √ • Cardinal Red 0.42 0.84 0.41 45 √ √ √ • Charcoal 0.28 0.84 0.28 27 √ √ √ • Cityscape 0.37 0.85 0.34 39 √ √ √ √ • Colonial Red 0.34 -

Wildflower in Focus: Cardinal Flower
throated hummingbirds. Their vivid color is the traditional color of the robes worn by Roman Wildflower in Focus Catholic Cardinals - and thus the name. Their hue is also similar to the color of the native male bird Text by Melanie Choukas-Bradley that shares the name cardinal. Growing in wet Artwork by Tina Thieme Brown meadows, springs, freshwater marshes, and along streams, rivers, and ponds throughout the state, the Cardinal Flower cardinal flower is one of several members of the Lobelia cardinalis L. Lobelia genus native to Maryland and the only one Bellflower or Bluebell Family (Campanulaceae) with red flowers. Other Lobelias growing here [Some taxonomists place this genus in a separate have flowers ranging from blue to whitish-blue, family — the Lobelia Family (Lobeliaceae)] lilac, and rarely white. The cardinal flower blooms during the verdant days of mid to late summer and stays in bloom as autumn stirs. Specific Characteristics of the Cardinal Flower Flowers: Scarlet, irregular, 2-lipped, with a 2-lobed upper lip, a 3-lobed lower one and a tubular base. Sexual parts protrude in a beak-like fashion (see illustration). Flowers 1-2" long and wide in upright racemes. Five sepals are thin (almost hair-like). Leaves: Alternate, simple, lanceolate to oblong or narrowly ovate, tapered to apex and base, pubescent or glabrous. Toothed, often irregularly so (sometimes dentate). Short-petioled to sessile, 2-7" long. Height and Growth Habit: 1-5'; usually unbranched. Range: New Brunswick to Minnesota, south to the Gulf of Mexico. Herbal Lore: American Indians used the roots and leaves of this plant for a number of conditions, including syphilis, typhoid, fevers, headaches and rheumatism. -

TREMCO Color Chart
TREMCO Color Chart Our colors are deep, rich and true. Made of Valspar’s Fluropon® High Performance Hylar 5000®/Kynar 500® finish, they offer the ultimate in resistance against fading and weathering. In addition to our many standard colors, custom colors are also available. EXPRESS COLORS* Stone White l Bone White l Almond l Sandstone l Cityscape l Slate Gray l Sierra Tan l Medium Bronze l Dark Bronze l Clear Anodized STANDARD COLORS Charcoal l Midnight (Extra Dark) Matte Black Burgundy (Brandywine) Terra Cotta l Bronze Cardinal Red Sky Blue Interstate Blue Forest (Sherwood) Green Patina Green l (Brite Red) l (Roman Blue) l (Regal Blue) Hartford Green Musket Gray l Colonial Red l Evergreen l Mansard Brown l Granite (Ash Gray) l PREMIUM COLORS Classic Copper (Penny) l Silver Metallic l Champagne Metallic l Zinc l 24 GA GALVALUME ONLY Regal White Surrey Beige Patrician Bronze Buckskin Charcoal * = Also in Mill Finish l l = Energy Star Rated Tremco receives metal through multiple vendors. If a specific metal vendor is required for a project, please specify this at the time of order. TREMCO Color Chart QUICK REFERENCE GUIDE Color 24 ga. 22 ga. .040” .050” .063” Please Note Almond w w w w w Tremco receives metal through Bone White w w w w w multiple vendors. If a specific metal Burgundy (Brandywine) w w w vendor is required for a project, please specify this at the time of Cardinal Red (Brite Red) w w w order. Charcoal w w w w Express colors available in shorter lead times for w w w w Cityscape 24 ga., .040” and .050” only. -

WILDLIFE TRAFFICKING in BRAZIL Sandra Charity and Juliana Machado Ferreira
July 2020 WILDLIFE TRAFFICKING IN BRAZIL Sandra Charity and Juliana Machado Ferreira TRAFFIC: Wildlife Trade in Brazil WILDLIFE TRAFFICKING IN BRAZIL TRAFFIC, the wildlife trade monitoring network, is a leading non-governmental organisation working globally on trade in wild animals and plants in the context of both biodiversity conservation and sustainable development. © Jaime Rojo / WWF-US Reproduction of material appearing in this report requires written permission from the publisher. The designations of geographical entities in this publication, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of TRAFFIC or its supporting organisations concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. TRAFFIC David Attenborough Building, Pembroke Street, Cambridge CB2 3QZ, UK. Tel: +44 (0)1223 277427 Email: [email protected] Suggested citation: Charity, S., Ferreira, J.M. (2020). Wildlife Trafficking in Brazil. TRAFFIC International, Cambridge, United Kingdom. © WWF-Brazil / Zig Koch © TRAFFIC 2020. Copyright of material published in this report is vested in TRAFFIC. ISBN: 978-1-911646-23-5 UK Registered Charity No. 1076722 Design by: Hallie Sacks Cover photo: © Staffan Widstrand / WWF This report was made possible with support from the American people delivered through the U.S. Agency for International Development (USAID). The contents are the responsibility of the authors and do not necessarily -

Variety Shade Cultivar Color Price Achillea Moonshine Canary Yellow 5.99 Achillea Saucy Seduction Hot Pink 5.99 Aconitum Fischeri Lg
Variety Shade Cultivar Color Price Achillea Moonshine Canary yellow 5.99 Achillea Saucy Seduction hot pink 5.99 Aconitum fischeri lg. purple blue hooded fl. 7.95 Ajuga Burgundy Glow reddish purple fol. White and pink 5.99 Ajuga Catlin's Giant bronze foliage 4.99 Ajuga Chocolate Chip chocolate foliage 5.99 Ajuga Pink Lightening crinkled light green/cream edges 5.99 Alcea Las Vegas mix 5.99 Alcea Mars Magic bright red 5.99 Alcea Mallow Zebrina pink flowers/purplish veins 5.99 Alchemilla Yes Gold Strike yellow gold flowers 5.99 Allium Millenium 2" round, bright rosy purple 7.99 Alyssum Golden Spring Bright yellow 5.99 Alyssum Saxatile Summit bright yellow 5.99 Anemone pulsatilla violet 5.99 Anemone rubra red 5.99 Anemone sylvestris white 5.99 Aquilegia Yes Swan Series Blue & White blue and white 5.99 Aquilegia Yes Swan Series Burgundy and White rich burgundy and white 5.99 Aquilegia Yes Swan Series Pink and Yellow lt. pink outer and yellow inner 5.99 Aquilegia Yes Swan Series Violet and White violet and white 5.99 Aquilegia Yes Swan Series Yellow yellow and cream 5.99 Arabis Snowfix small white 5.99 Aralia Yes Sun King bright gold leaves 10.99 Aralia Yes Sun King bright gold leaves 12.99 Arenaria Blizzard Compact cup shaped white/yellow flowers 5.99 Armeria Maritima Morning Star Deep Rose rose pink 5.99 Artemesia Silver Mound silver 5.99 Aruncus Yes Dwarf Goatsbeard white 5.99 Aruncus Yes Goatsbeard white 5.99 Asclepias Butterfly Flower orange flr 2-3' 6.99 Asclepias Butterfly Flower orange flr 2-3' 5.99 Asclepias Cinderella incarnata pink -

Brazilian Cardinal by Edith Pendleton, Ph.D., Fort Meyers, Florida
Domestic Breeding of the Brazilian Cardinal by Edith Pendleton, Ph.D., Fort Meyers, Florida ittle has been written and even less ing and stress, and those that survived demeanor. On the other hand, once a bird documented about the domestic often bore the scars of combat, including reaches puberty, it will fight for and L breeding habits of the Red-crested missing toes and eyes, mauled feet, or defend its hen to the death. A bird can be (Brazilian) Cardinal. With encouragement amputations. fully scalped and left bleeding to death in from Kenneth Reininger, Curator of Birds the course of 10 minutes; a bird can 10 e at the North Carolina Zoological Park, pli one or more toes, or an eye, in five min vate aviculturalists have begun a coopera '13razifian Caraina[s tliri[[ utes. Two birds housed together may be tive infOImation exchange with zoos on to tlie company of tlieir own congenial at dawn, but mortally maimed behalf of this beleaguered softbill. Only {in" antI aViary observe by afternoon, should spring fever strike six zoos nationwide successfully reared ana imitate one anotlier. them. For that reason, never leave two Red-crested Cardinals in 1992, from a unsexed birds nearing puberty together, or pool of 86. There were 62 birds in zoos in even housed side by side, from February 1987, and 52 fi ve years earlier, in 1982, Pairing and Sexing Birds through August, the breeding months, perhaps reflecting the growing trend Brazilian Cardinals thrill to the compa unless you have every guarantee they are toward walk-in rainforest exhibits. -

Federal Register/Vol. 70, No. 49/Tuesday, March 15, 2005/Notices
12710 Federal Register / Vol. 70, No. 49 / Tuesday, March 15, 2005 / Notices values or resources that would be ADDRESSES: The complete file for this Commission; North Dakota Game and considered significant. notice is available for inspection, by Fish Department; Oklahoma Department Based upon this preliminary appointment (contact John L. Trapp, of Wildlife Conservation; Pennsylvania determination, we do not intend to (703) 358–1714), during normal Game Commission; Rhode Island prepare further NEPA documentation. business hours at U.S. Fish and Wildlife Division of Fish and Wildlife; South We will consider public comments in Service, 4501 North Fairfax Drive, Room Dakota Department of Game, Fish, and making the final determination on 4107, Arlington, Virginia. Parks; Vermont Department of Fish and whether to prepare such additional SUPPLEMENTARY INFORMATION: Wildlife; Virginia Department of Game and Inland Fisheries; Wisconsin documentation. What Is the Authority for This Notice? This notice is provided pursuant to Department of Natural Resources; and section 10(c) of the Act. We will Migratory Bird Treaty Reform Act of Wyoming Game and Fish Department), evaluate the permit application, the 2004 (Division E, Title I, Sec. 143 of the 11 nonprofit organizations representing proposed Plan, and comments Consolidated Appropriations Act, 2005, bird conservation and science interests submitted thereon to determine whether Pub. L. 108–447). (American Bird Conservancy— submitted on behalf of 10 constituent the application meets the requirements What Is the Purpose of This Notice? organizations; Atlantic Flyway of section 10(a) of the Act. If the The purpose of this notice is to make requirements are met, we will issue a Council—representing 17 States, 7 the public aware of the final list of ‘‘all Provinces, Puerto Rico, and the U.S. -

Cardinal Health Sterilization Wraps (Continued)
Cardinal Health™ Sterilization Wrap Your trusted source for ease and assurance Everything you need, wrapped in a single solution Every day, you’re tasked to do more with less. To improve productivity with less time, budget and staff. And do it all while you strive to keep your patients safe and improve the quality of care. That’s why we created a new choice in sterilization wrap: a full selection of products that are durable, reliable and cost effective. All the selection you want Whatever your clinicians need, now you have a single product line that can take care of it all. Choose the wrap that best meets your goals of maximizing efficiency, safety and quality of care. Two Color: Single Layer: The blue and green layers Supports sequential help make it fast and easy wrapping methodologies to differentiate, identify and inspect trays • Made from SMS polypropylene, non-woven material for strength and sterility maintenance. • Designed with a simple, “cross-stitch” bond pattern for clear, visual feedback of breaches that may occur during handling, storage and/or sterilization. • Available in six basis weights and a wide range of sizes to meet the needs of today’s Central Sterile Department. Cardinal Health™ Sterilization Wraps Two Color Two Color Single Layer Catalog no. Size/Dimensions Case qty. Catalog no. Case qty. • Made from two layers of SMS — one CH1G0012 12 x 12 in. (30 x 30cm) 480 CH110012 1000 layer blue, the other green — bonded CH1G0015 15 x 15 in. (38 x 38cm) 480 CH110015 1000 CH1G0018 18 x 18 in. (45 x 45cm) 480 CH110018 1000 together along opposing sides CH1G0020 20 x 20 in. -

Paroaria Baeri and P. Gularis)
Emu - Austral Ornithology ISSN: 0158-4197 (Print) 1448-5540 (Online) Journal homepage: http://www.tandfonline.com/loi/temu20 Mixing the waters: a linear hybrid zone between two riverine Neotropical cardinals (Paroaria baeri and P. gularis) Juan I. Areta, Túlio Dornas, Guy M. Kirwan, Lucas Eduardo Araújo-Silva & Alexandre Aleixo To cite this article: Juan I. Areta, Túlio Dornas, Guy M. Kirwan, Lucas Eduardo Araújo-Silva & Alexandre Aleixo (2017) Mixing the waters: a linear hybrid zone between two riverine Neotropical cardinals (Paroaria baeri and P. gularis), Emu - Austral Ornithology, 117:1, 40-50 To link to this article: http://dx.doi.org/10.1080/01584197.2016.1266447 View supplementary material Published online: 16 Feb 2017. Submit your article to this journal Article views: 78 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=temu20 Download by: [190.231.157.48] Date: 21 March 2017, At: 18:47 EMU AUSTRAL ORNITHOLOGY, 2017 VOL. 117, NO. 1, 40–50 http://dx.doi.org/10.1080/01584197.2016.1266447 Mixing the waters: a linear hybrid zone between two riverine Neotropical cardinals (Paroaria baeri and P. gularis) Juan I. Areta a, Túlio Dornasb, Guy M. Kirwanc,d, Lucas Eduardo Araújo-Silvae and Alexandre Aleixof aInstituto de Bio y Geociencias del Noroeste Argentino – IBIGEO-CONICET, Salta, Argentina; bPrograma de Pós-Graduação Rede Bionorte – Área Biodiversidade e Conservação & Grupo de Pesquisa em Ecologia e Conservação das Aves,