Running the Trophic Gauntlet: Empirical Support for Reduced Foraging Success in Juvenile Salmon in Tidally Mixed Coastal Waters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Raincoast & LOS Application for Leave TM Reconsideration Vol 1
Court File No. _________ FEDERAL COURT OF APPEAL BETWEEN: RAINCOAST CONSERVATION FOUNDATION and LIVING OCEANS SOCIETY Applicants and ATTORNEY GENERAL OF CANADA and TRANS MOUNTAIN PIPELINE ULC Respondents APPLICANTS’ MOTION RECORD Motion for leave to apply for judicial review of Order in Council, P.C. 2019-820 made by the Governor in Council under subsection 54(1) of the National Energy Board Act VOLUME 1 OF 4 ANY RESPONDENT WISHING TO FILE A MOTION RECORD IN RESPONSE TO THIS MOTION FOR LEAVE MUST DO SO WITHIN TEN (10) DAYS OF BEING SERVED. REFER TO THE PRACTICE DIRECTION INCLUDED IN THIS MOTION RECORD AT PAGES 75 - 80 Dyna Tuytel & Margot Venton Counsel for the Applicants 800, 744 – 4 Avenue SW Calgary, AB T2P 3T4 Phone: 403 705-0202 Fax: 403-452-6574 TO: FEDERAL COURT OF APPEAL 3rd Floor, 635 – 8 Avenue SW Calgary, AB T2P 3M3 AND TO: ATTORNEY GENERAL OF CANADA c/o Department of Justice Canada Suite 601. 606 – 4 Street SW Calgary, AB T2P 1T1 Tel: 403 292-6813 Fax: 403 299-3507 TRANS MOUNTAIN PIPELINE ULC c/o Osler, Haskin & Harcourt LLP Suite 2500, TransCanada Tower 450 – 1 Street Sw Calgary, AB T2P 5H1 Tel: (403) 260-7003/7038 Fax: (403) 260-7024 NATIONAL ENERGY BOARD 517 – 10 Avenue SW Calgary. AB T2R 0A8 Tel: 403 292-4800 Fax: 403 292-5503 TABLE OF CONTENTS VOLUME 1 Tab Document Page 1 Notice of Motion 1 2 Order in Council, P.C. 2019-820, published in the Canada Gazette, Part I, 9 dated June 22, 2019 3 Practice Direction of Sharlow J.A., Applications for leave to apply for 75 judicial review under subsection 55(1) of the National Energy -

Feed Grain Transportation and Storage Assistance Regulations
CANADA CONSOLIDATION CODIFICATION Feed Grain Transportation and Règlement sur l’aide au Storage Assistance Regulations transport et à l’emmagasinage des céréales C.R.C., c. 1027 C.R.C., ch. 1027 Current to November 21, 2016 À jour au 21 novembre 2016 Published by the Minister of Justice at the following address: Publié par le ministre de la Justice à l’adresse suivante : http://laws-lois.justice.gc.ca http://lois-laws.justice.gc.ca OFFICIAL STATUS CARACTÈRE OFFICIEL OF CONSOLIDATIONS DES CODIFICATIONS Subsections 31(1) and (3) of the Legislation Revision and Les paragraphes 31(1) et (3) de la Loi sur la révision et la Consolidation Act, in force on June 1, 2009, provide as codification des textes législatifs, en vigueur le 1er juin follows: 2009, prévoient ce qui suit : Published consolidation is evidence Codifications comme élément de preuve 31 (1) Every copy of a consolidated statute or consolidated 31 (1) Tout exemplaire d'une loi codifiée ou d'un règlement regulation published by the Minister under this Act in either codifié, publié par le ministre en vertu de la présente loi sur print or electronic form is evidence of that statute or regula- support papier ou sur support électronique, fait foi de cette tion and of its contents and every copy purporting to be pub- loi ou de ce règlement et de son contenu. Tout exemplaire lished by the Minister is deemed to be so published, unless donné comme publié par le ministre est réputé avoir été ainsi the contrary is shown. publié, sauf preuve contraire. -

Recovery Strategy for the Northern and Southern Resident Killer Whales (Orcinus Orca) in Canada
Species at Risk Act Recovery Strategy Series Recovery Strategy for the Northern and Southern Resident Killer Whales (Orcinus orca) in Canada KILLER WHALE Photo: G. Ellis March 2008 About the Species at Risk Act Recovery Strategy Series What is the Species at Risk Act (SARA)? SARA is the Act developed by the federal government as a key contribution to the common national effort to protect and conserve species at risk in Canada. SARA came into force in 2003 and one of its purposes is “to provide for the recovery of wildlife species that are extirpated, endangered or threatened as a result of human activity.” What is recovery? In the context of species at risk conservation, recovery is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of the species’ persistence in the wild. A species will be considered recovered when its long-term persistence in the wild has been secured. What is a recovery strategy? A recovery strategy is a planning document that identifies what needs to be done to arrest or reverse the decline of a species. It sets goals and objectives and identifies the main areas of activities to be undertaken. Detailed planning is done at the action plan stage. Recovery strategy development is a commitment of all provinces and territories and of three federal agencies — Environment Canada, Parks Canada Agency, and Fisheries and Oceans Canada — under the Accord for the Protection of Species at Risk. Sections 37–46 of SARA (http://www.sararegistry.gc.ca/the_act/default_e.cfm) outline both the required content and the process for developing recovery strategies published in this series. -

Poster (7.573Mb)
T h e C o a s t - Insular Boundary Revisited: Mid-Jurassic ductile deformation on Quadra Island, British Columbia Sandra Johnstone [email protected]; Vancouver Island University, 900 Fifth Street, Nanaimo British Columbia, V9R 5S5 Project Overview Relationship to Strongly deformed basalt dikes are boudined and transposed parallel Sample Description Ductile Strongly Deformed Dike to bedding. Mineralogy of OB-14-26 is dominated by subhedral laths 35 Post-Deformation Intrusions The boundary between the Insular and Coast geomorphological belts of the North American Cordillera is well exposed at Open Deformation of strongly sericitized plagioclase averaging 0.5mm (70%), with 20% Samples OB-14-24 and OB-14-27 are both intrusive phases that cross-cut ductile deformation struc- N Bay, on Quadra Island, British Columbia. On the southwestern side of the boundary at Open Bay is the upper Triassic-aged actinolite that may be an alteration product of hornblende. Unfortu- N Basaltic Pre– or early syn- 83 tures. OB-14-24 is an ~5m wide unfoliated plagioclase-phyric dacite dike. OB-14-24 zircons are zoned Quatsino limestone and Karmutsen basalts of the Wrangellia terrane. On the northeastern side of the boundary are intrusive OB-14-26 nately only one zircon was recovered from Sample OB-14-26. This andesite pod deformation 65 single zircon yielded a concordant age of 157.6 ± 8.9 Ma. euhedral to subhedral crystals and some fragments; nineteen crystals yield an age of 158.7 ± 0.8 Ma. phases of the Coast Batholith. At Open Bay the Quatsino limestone (Upper Triassic) has been subjected to strong ductile defor- N Folded mation resulting in re-folded transposed bedding. -

Staff Report
STAFF REPORT DATE: September 3, 2021 FILE: 0540-04 EASC TO: Chair and Directors, Electoral Area Services Committee FROM: Dave Leitch Chief Administrative Officer RE: COMMUNITY RESILIENCY INVESTMENT - GRANT OPPORTUNITY PURPOSE To consider an application to the Community Resiliency Investment grant program of the Union of BC Municipalities (UBCM) to further the Regional District’s efforts to reduce wildfire risks. EXECUTIVE SUMMARY The Community Resiliency Investment (CRI) grant is a provincial program intended to reduce the risk and impact of wildfires on communities in BC. The general goal of FireSmart is to encourage communities and citizens to adopt and conduct FireSmart practices to mitigate the negative impacts of wildfire to public and private property assets. The program can contribute up to 100% of the cost of eligible activities provided the application has a Council or Board resolution indicating support for the proposed activities and a willingness to provide overall grant management. Regional Districts may submit a single application for eligible, collaborative projects that include multiple electoral areas. The maximum base funding for fuel management is $50,000 plus up to $50,000 for FireSmart activities for each electoral area. All local governments (municipalities and regional districts) and First Nations (bands and Treaty First Nations) in BC are eligible to apply. Eligible applicants may submit one application per intake. The deadline for the next intake of applications is October 8, 2021. It is proposed that an application be submitted by the Regional District to undertake a number of FireSmart activities in each electoral area as outlined below in detail. If approved, it is anticipated that 100% of the total costs of the FireSmart activities would be covered by the grant award. -

Baseline Inventory – Village of Cumberland Forest Lands
PREPARED FOR THE VILLAGE OF CUMBERLAND by Tim Ennis Latitude Conservation Solutions Company BASELINE INVENTORY – VILLAGE OF CUMBERLAND FOREST LANDS August 2019 Baseline Inventory - Village of Cumberland Forest Lands Executive Summary The Corporation of the Village of Cumberland (the Village) owns 229.5 hectares (567.1 acres) of land in seven parcels within the municipal boundaries of the Village of Cumberland which it manages for the storage and conveyance of drinking water (the Lands). The Lands include the Stevens Lake Reservoir, Hamilton Lake Reservoir, #2 Reservoir and Henderson Lake. Each of these are connected sequentially by Cumberland Creek, a tributary of Perseverance Creek. The Lands also include the Allen Lake Reservoir which drains directly into Perseverance Creek. Approximately 65 hectares (160 acres) of the Lands (28%) are outside of the Cumberland Creek and Allen Lake drainages, and therefore do not contribute to the Village’s drinking water supply watersheds. These areas primarily drain through an unnamed creek (locally known as Lookout Creek) and thence into Perseverance Creek downstream of the Village’s water supply infrastructure. The Lands are located at the northern tip of the Beaufort Range in the Comox Valley, extending from roughly 650 meters above sea-level (masl) in the vicinity of Steven’s Lake to 300 masl at the downstream end of Cumberland Creek and in the vicinity of Allen Lake are approximately 250 masl. The topography is varied, and includes depressions and the toe of slopes, deeply incised canyons, and rolling hills. The Lands occur within the Coastal Western Hemlock biogeoclimatic zone and span three subzones/variants. -

NOAA Technical Memorandum NMFS FINWC-122
NOAA Technical Memorandum NMFS FINWC-122 A Listing oi pacific coast JfD"ri Spawnins Streams and Hatcheries producing Chinook and Coho Salmon with Estimates on Numbers of Spawners and Data on Hatchery Releases by Roy J. Wahle and Rager E . Parson September 1987 US. DEPARTMENT OF COMMERCE National Ocrranic and Atmospheric Administration National Marine Fisheries Service This TM series is uoed for documentation and timly communication of plhinery resul.rs, interh reports, or s cia1 purpase Information, and has nM received mmpbb fomi review, editorial conrol, or detailed editing. A LISTING OF PACIFIC COAST SPAWNING STREAMS AND HATCHERIES PRODUCING CHINOOK AND COHO SALMON with Estimates on Numbers of Spawners and Data on Hatchery Releases Roy J. Wahleu and Roger E. pearsonu UPacific Marine Fisheries Commission 2000 S.W. First Avenue Metro Center, Suite 170 Port1and, OR 97201-5346 Present address: 8721 N.E. Bl ackburn Road Yamhill, OR 97148 2/(CO-author deceased ) Northwest and Alaska Fisheries Center National Marine Fisheries Service National Oceanic and Atmospheric Admini stration 2725 Montl ake Boulevard East Seattle, WA 98112 September 1987 This document is available to the public through: National Technical Information Service U.S. Department of Commerce 5285 Port Royal Road Springfield, VA 22161 iii ABSTRACT Information on chinook, Oncorhynchus tshawytscha, and coho, -0. kisutch, salmon spawning streams and hatcheries along the west coast of North Ameriica was compiled following extensive consultations with fishery managers and biologists and thorough review of pub1 ished and unpublished information. Included are a listing of all spawning streams known as of 1984-85, estimates of the annual number of spawners observed in the streams, and data on the annual production of juveni le chinook and coho salmon at a1 1 hatcheries. -

Code Search Results
ECAS Code List Code Table Code Value Description Where Used in Application Notes ADS_INSECT_SPECIES_CODE MPB Mountain Pine Beetle Interior UNK Unknown ADS_SPECIES_DAMAGE_CATGRY_CODE G Green Interior Expires on Dec 1, 2007 GA Green Attack RA Red Attack YA Gray Attack DP Dead Potential Expires on Dec 1, 2007 Ads_Location_Code CARV Campbell River Coast CHWK Chilliwack HOUS Houston MERR Merritt NANA Nanaimo PRRU Prince Rupert TERR Terrace VANC Vancouver VICT Victoria Appraisal_Amendment_Type_Code ADD Addition Coast DEL Deletion Appraisal_Category_Code Common N Initial ADS R Reappraisal D Redetermination Expires on Aug 1, 2013 P Post-Harvest ADS Effective on Apr 1, 2019 Apprsl_Certification_Type_Code R Reviewed Common S Supervised P Personally Prepared Appraisal_Culvert_Type_Code W Wooden Coast M Metal Coast T Tabular Interior Appraisal_Document_Type_Code BR Detailed Engineering - Bridge Repairs Coast Expired Dec 15, 2019 CAF Cruise - Cruise Analysis Form Coast CEF1 NDC Form #1 Coast CEF2 NDC Form #2 Coast CEF3 NDC Form #3 Coast CEF4 NDC Form #4 Coast CEF5 NDC Form #5 Coast CEF6 NDC Form #6 Coast CEF7 NDC Form #7 Coast CEF8 NDC Form #8 Coast CEF9 NDC Form #9 Coast CEF10 NDC Form #10 Coast CEF11 NDC Form #11 Coast CEF12 NDC Form #12 Coast CEF13 NDC Form #13 Coast CEF14 NDC Form #14 Coast CEF15 NDC Form #15 Coast CEF16 NDC Form #16 Coast CEF17 NDC Form #17 Coast CEF18 NDC Form #18 Coast CEF19 NDC Form #19 Coast CEF20 NDC Form #20 Coast SOFZ Specified Operations - Fibre Recovery Zone Coast SOMS Specified Operations - Miscellaneous Coast DCDA -

2011 Final Killer Whale Recovery Strategy
Final - Amended Species at Risk Act Recovery Strategy Series Recovery Strategy for the Northern and Southern Resident Killer Whales (Orcinus orca) in Canada Killer Whale Photo: G. Ellis August 2011 About the Species at Risk Act Recovery Strategy Series What is the Species at Risk Act (SARA)? SARA is the Act developed by the federal government as a key contribution to the common national effort to protect and conserve species at risk in Canada. SARA came into force in 2003 and one of its purposes is “to provide for the recovery of wildlife species that are extirpated, endangered or threatened as a result of human activity.” What is recovery? In the context of species at risk conservation, recovery is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of the species’ persistence in the wild. A species will be considered recovered when its long-term persistence in the wild has been secured. What is a recovery strategy? A recovery strategy is a planning document that identifies what needs to be done to arrest or reverse the decline of a species. It sets goals and objectives and identifies the main areas of activities to be undertaken. Detailed planning is done at the action plan stage. Recovery strategy development is a commitment of all provinces and territories and of three federal agencies — Environment Canada, Parks Canada Agency, and Fisheries and Oceans Canada — under the Accord for the Protection of Species at Risk. Sections 37–46 of SARA (http://www.sararegistry.gc.ca/the_act/default_e.cfm) outline both the required content and the process for developing recovery strategies published in this series. -
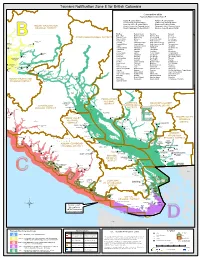
Tsunami Notification Zone E for British Columbia
Tsunami Notification Zone E for British Columbia Communities within Tsunami Notification Zone E Capital Regional District Nanaimo Regional District Comox Valley Regional District Powell River Regional District Cowichan Valley Regional District Strathcona Regional District MOUNT WADDINGTON Greater Vancouver Regional District Squamish-Lillooet Regional District REGIONAL DISTRICT CARIBOOMount Waddington REGIONAL Regional DistrictDISTRICTSunshine Coast Regional District Big Bay Galiano Island Nanaimo Sayward Bones Bay Gambier Island Nanoose Bay Sechelt STRATHCONA REGIONAL DISTRICT Brittania Beach Garden Bay Nelson Island Secret Cove Bowen Island Gibsons New Westminster Semiahmoo Buckley Bay Halalt North Saanich Shelter Point B Dzawada'enuxw (636) Burnaby Hardwicke Island North Vancouver City Sliammon Gwawaenuk Tribe (627) Kingcome Campbell River Hornby Island North Vancouver DM Snuneymuxvw t Gold le Cedar K'omoks (Comox) Oyster River Squamish n Bridge I t Central Saanich Karlukwees Parksville Squamish DM Sullivan Knigh Bay Chemainus Klahoose Pauquachin Surrey Comox Kwiakah Penelakut Stz`uminusPioneer Mine Thompson Cortez Island Ladner Pender Island Texada Island Sound Seton Courtenay Ladysmith Port Mellon Thompson Sound Portage Echo Bay Cowichan Lang Bay Port Moody Thormanby Island Kwicksutaineuk-ah- Cowichan Bay Langford Port Neville Tlowitsis kwaw-ah-mish (625) Cracroft Lantzville Powell River Tsartlip Crofton Lasqueti Island Quadra Island Tsawwaassen Minstrel Cumberland Lyackson Qualicum Tseil-Waututh Mamalilikulla- Island Qwe'Qwa'Sot'Em -
Killer Whale Recovery Strategy
Proposed Species at Risk Act Recovery Strategy Series Recovery Strategy for the Northern and Southern Resident Killer Whales (Orcinus orca) in Canada Killer Whale Photo: G. Ellis Original publication 2008 1st amendment 2011 2nd amendment 2018 Recovery Strategy for the Northern and Southern Resident Killer Whales in Canada [Proposed] 2018 Recommended citation: Fisheries and Oceans Canada. 2018. Recovery Strategy for the Northern and Southern Resident Killer Whales (Orcinus orca) in Canada [Proposed]. Species at Risk Act Recovery Strategy Series, Fisheries & Oceans Canada, Ottawa, x + 84 pp. For copies of the recovery strategy, or for additional information on species at risk, including Committee on the Status of Endangered Wildlife in Canada (COSEWIC) Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the SAR Public Registry. Cover illustration: Graeme Ellis, Fisheries & Oceans Canada Également disponible en français sous le titre : « Programme de rétablissement des épaulards résidents (Orcinus orca) du nord et du sud au Canada» © Her Majesty the Queen in Right of Canada, represented by the Minister of Fisheries & Oceans, 2018. All rights reserved. ISBN Catalogue no. Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source. ii Recovery Strategy for the Northern and Southern Resident Killer Whales in Canada [Proposed] 2018 Preface The federal, provincial, and territorial government signatories under the Accord for the Protection of Species at Risk (1996) agreed to establish complementary legislation and programs that provide for effective protection of species at risk throughout Canada. Under the Species at Risk Act (S.C. 2002, c.29) (SARA), the federal competent ministers are responsible for the preparation of a recovery strategy for species listed as extirpated, endangered, or threatened and are required to report on progress five years after the publication of the final document on the Species at Risk Public Registry. -
A2 Marine Mammal Guidelines and Marine Protected Areas
Notices to Mariners 1 to 46 Section A – Aids to Navigation and Marine Safety A2 Marine Mammal Guidelines and Marine Protected Areas 5 General Guidelines for Aquatic Species at Risk and Important Marine Mammal Areas Fisheries and Oceans Canada is responsible for ensuring the protection and conservation of aquatic species at risk listed under the Species at Risk Act (SARA) (including listed marine mammals), and for protecting their critical habitat once identified. Critical habitat is defined in SARA section 2(1) as “…the habitat that is necessary for the survival or recovery of a listed wildlife species and that is identified as the species’ critical habitat in the recovery strategy or in an action plan for the species.” SARA defines habitat for aquatic species at risk as “… spawning grounds and nursery, rearing, food supply, migration and any other areas on which aquatic species depend directly or indirectly in order to carry out their life processes, or areas where aquatic species formerly occurred and have the potential to be reintroduced”. Under SARA, it is an offence to kill, harm, harass, capture, take , possess, collect, buy, sell or trade any SARA-listed extirpated, endangered or threatened animal or any part or derivative of an individual to damage or destroy the residences of its individuals. It is also prohibited to destroy critical habitat, once it has been identified in a recovery plan or action plan and is legally protected. These prohibitions do not apply to species listed as special concern. Individuals who contravene the provisions of SARA may be found guilty of an offence and liable for a fine or penalty pursuant to section 97 of SARA.