Jacobaea Through the Eyes of Spectroscopy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Senecio Jacobaea in Northern California, an Enduring Success
ENTOMOPHAGA 35 (I), 1990, 71-77 BIOLOGICAL CONTROL OF SENECIO JACOBAEA IN NORTHERN CALIFORNIA, AN ENDURING SUCCESS R. W. PEMBERTON (t) & C. E. TURNER Rangeland Insect Lab, USDA-ARS, Montana State University, Bozeman, Montana 59717 Biological Control of Weeds Western Regional Research Center USDA-ARS, Albany, California 94710 Seneciojacobaea, a poisonous weed from Eurasia, was brought under successful biological control in the Ft. Bragg, California area by 1976, through the combined action of the defoliating cinnabar moth (Tyriajacobaeae) and a root feeding flea beetle (Longitarsusjacobaeae). In 1987, 4 previously infested Ft. Bragg sites (3 sites where control had been documented and another unstudied site) were examined. Senecio jacobaea densities at these sites were 0.0, 0.0, 0.01 and 0.18 plants/m 2, indicating both continued and improved control of the weed. The flea beetle and the cinnabar moth both persist at the sites, despite very low numbers of S.jacobaea plants. The control of S. jacobaea has resulted in the return of near natural vegetation at the 2 coastal prairie sites and regained productivity at the 2 pasture sites. KEY-WORDS : Biological control, cinnabar moth, Longitarsusjacobaeae, poiso- nous plant, Seneciojacobaea, tansy ragwort, Tyria jacobaeae. INTRODUCTION THE WEED PROBLEM Tansy ragwort, (Senecio jacobaea L. : Asteraceae) is a biennial or short lived perennial herb that is native to Europe eastward to Siberia (Harper & Wood, 1957). The plant is an introduced weed in New Zealand, Australia, South Africa, Argentina, Brazil, Canada, and the United States (Holm et al., 1979). Tansy ragwort contains toxic pyrrolizidine alkaloids which cause liver damage and death to cattle and horses that ingest it (Kingsbury, 1964 ; Harris et aL, 1984). -

Stace Edition 4: Changes
STACE EDITION 4: CHANGES NOTES Changes to the textual content of keys and species accounts are not covered. "Mention" implies that the taxon is or was given summary treatment at the head of a family, family division or genus (just after the key if there is one). "Reference" implies that the taxon is or was given summary treatment inline in the accounts for a genus. "Account" implies that the taxon is or was given a numbered account inline in the numbered treatments within a genus. "Key" means key at species / infraspecific level unless otherwise qualified. "Added" against an account, mention or reference implies that no treatment was given in Edition 3. "Given" against an account, mention or reference implies that this replaces a less full or prominent treatment in Stace 3. “Reduced to” against an account or reference implies that this replaces a fuller or more prominent treatment in Stace 3. GENERAL Family order changed in the Malpighiales Family order changed in the Cornales Order Boraginales introduced, with families Hydrophyllaceae and Boraginaceae Family order changed in the Lamiales BY FAMILY 1 LYCOPODIACEAE 4 DIPHASIASTRUM Key added. D. complanatum => D. x issleri D. tristachyum keyed and account added. 5 EQUISETACEAE 1 EQUISETUM Key expanded. E. x meridionale added to key and given account. 7 HYMENOPHYLLACEAE 1 HYMENOPHYLLUM H. x scopulorum given reference. 11 DENNSTAEDTIACEAE 2 HYPOLEPIS added. Genus account added. Issue 7: 26 December 2019 Page 1 of 35 Stace edition 4 changes H. ambigua: account added. 13 CYSTOPTERIDACEAE Takes on Gymnocarpium, Cystopteris from Woodsiaceae. 2 CYSTOPTERIS C. fragilis ssp. fragilis: account added. -

Asteraceae, Senecioneae), with Nomenclatural Notes on This Name
Phytotaxa 234 (3): 271–279 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2015 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.234.3.8 Molecular and cytogenetic confirmation of the hybrid origin of Jacobaea ×mirabilis (Asteraceae, Senecioneae), with nomenclatural notes on this name GEMMA MAS DE XAXARS1, ALAIN FRIDLENDER2, TERESA GARNATJE3 & JOAN VALLÈS1 1 Laboratori de Botànica - Unitat Associada CSIC, Facultat de Farmàcia, Universitat de Barcelona. Avinguda Joan XXIII s/n, 08028 Barcelona (Catalonia, Spain); [email protected], [email protected] 2 Faculté des Sciences, Université de Provence - AMU, case 75, 3 place Victor Hugo, 13331 Marseille Cedex 3 (France); [email protected] 3 Institut Botànic de Barcelona (IBB-CSIC-ICUB), Passeig del Migdia s/n, 08038 Barcelona (Catalonia, Spain); [email protected] Abstract Jacobaea ×mirabilis is a hybrid taxon, formerly included in the genus Senecio, originating from J. adonidifolia and J. leu- cophylla as progenitors. It inhabits a few French mountain locations in the Pyrenees and in the Massif Central. In this paper, we characterize the hybrid and its parental taxa using molecular phylogenetic and cytogenetic (genome size) methods. These data are useful to confirm the hybrid status of the studied taxon. Additionally, we clarify nomenclatural issues connected with this species and fulfil the conditions for valid publication of its name in Jacobaea. The name of this hybrid is also lectotypified. Key words: genome size, homoploid hybridization, nomenclatural combination, phylogeny, Senecio ×mirabilis, typification Introduction Hybridization has classically been and is still recognized as a powerful evolutionary mechanism in plants, particularly in diversification and speciation through genetic exchange (Anderson 1949; Stebbins 1959; Arnold 2006). -
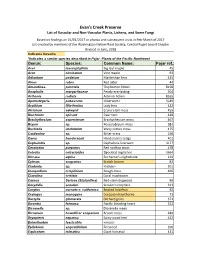
Evans-Creek-Plant-List.Pdf
Evan's Creek Preserve List of Vascular and Non‐Vascular Plants, Lichens, and Some Fungi Based on findings on 01/01/2017 or photos and subsequent visits in Feb‐March of 2017 List created by members of the Washington Native Plant Society, Central Puget Sound Chapter Revised in June, 2018 Indicates invasive *Indicates a similar species described in Pojar -Plants of the Pacific Northwest Genus: Species: Common Name: Pojar ref.: Acer macrophyllum Big leaf maple 45 Acer circinatum Vine maple 93 Adiantum pedatum Maidenhair fern 425 Alnus rubra Red alder 44 Amandinea punctata Tiny button lichen B158 Anaphalis margaritaceae Pearly everlasting 304 Arthonia radiata Asterisk lichen B165 Apometzgeria pubescens <liverwort> SL49 Arythium filix-femina Lady fern 422 Atrichum selwynii Crane's‐bill moss 455 Blechnum spicant Deer fern 420 Brachythecium asperrimum Brachythecium moss 467 Bryum sp. Rossulobryum moss S82 Buckiella undulatum Wavy cotton moss 475 Cardamine sp. Bitter‐cress 146 Carex hendersonii Henderson's sedge 401 Cephalozia sp. Cephalozia liverwort SL77 Ceratodon purpurus Red rooftop moss 478 Cetrelia cetrarioides Speckled rag lichen M64 Circaea alpina Enchanter's nightshade 210 Cytisus scoparius Scotch broom 83 Cladonia sp. <lichen> 501 Claopodium crispifoium Rough moss 466 Clavulina cristata Coral mushroom Cornus Sericea (Stolonifera) Red‐stem dogwood 90 Corydalis scouleri Scouler's corydalis 313 Corylus cornuta v. californica Beaked hazelnut 92 Crategus monogyna European hawthorne 73 Dactylis glomerata Orchard grass 371 Dicentra formosa -

(Linaria Vulgaris) and Dalmatian Toadflax (Linaria
DISSERTATION VIABILITY AND INVASIVE POTENTIAL OF HYBRIDS BETWEEN YELLOW TOADFLAX (LINARIA VULGARIS) AND DALMATIAN TOADFLAX (LINARIA DALMATICA) Submitted by Marie F.S. Turner Department of Soil and Crop Sciences In partial fulfillment of the requirements For the Degree of Doctor of Philosophy Colorado State University Fort Collins, Colorado Fall 2012 Doctoral Committee: Advisor: Sarah Ward Christopher Richards David Steingraeber George Beck Sharlene Sing Copyright by Marie Frances Sundem Turner 2012 All Rights Reserved ABSTRACT VIABILITY AND INVASIVE POTENTIAL OF HYBRIDS BETWEEN YELLOW TOADFLAX (LINARIA VULGARIS) AND DALMATIAN TOADFLAX (LINARIA DALMATICA) Although outcomes of hybridization are highly variable, it is now considered to play an important role in evolution, speciation, and invasion. Hybridization has recently been confirmed between populations of yellow (or common) toadflax (Linaria vulgaris) and Dalmatian toadflax (Linaria dalmatica) in the Rocky Mountain region of the United States. The presence of hybrid toadflax populations on public lands is of concern, as both parents are aggressive invaders already listed as noxious weeds in multiple western states. A common garden experiment was designed to measure differences in quantitative (shoot length, biomass, flowering stems, seed capsule production) phenological (time of emergence, first flowering and seed maturity) and ecophysiological (photosynthesis, transpiration and water use efficiency (WUE)) traits for yellow and Dalmatian toadflax, F1 and BC1 hybrids, as well as natural field-collected hybrids from two sites. Genotypes were cloned to produce true replicates and the entire common garden was also replicated at two locations (Colorado and Montana); physiological data were collected only in Colorado. All genotypes grew larger and were more reproductively active in Colorado than in Montana, and hybrids outperformed parent taxa across vegetative and reproductive traits indicating heterosis. -

Identification and Functional Characterization of the First Two
Identification and functional characterization of the first two aromatic prenyltransferases implicated in the biosynthesis of furanocoumarins and prenylated coumarins in two plant families: Rutaceae and Apiaceae Fazeelat Karamat To cite this version: Fazeelat Karamat. Identification and functional characterization of the first two aromatic prenyl- transferases implicated in the biosynthesis of furanocoumarins and prenylated coumarins in two plant families: Rutaceae and Apiaceae. Agronomy. Université de Lorraine, 2013. English. NNT : 2013LORR0029. tel-01749560 HAL Id: tel-01749560 https://hal.univ-lorraine.fr/tel-01749560 Submitted on 29 Mar 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. AVERTISSEMENT Ce document est le fruit d'un long travail approuvé par le jury de soutenance et mis à disposition de l'ensemble de la communauté universitaire élargie. Il est soumis à la propriété intellectuelle de l'auteur. Ceci implique une obligation de citation et de référencement lors de l’utilisation de ce document. D'autre part, toute contrefaçon, plagiat, -

Asteraceae) from Turkey: Senecio Inops Boiss
Turk J Bot 33 (2009) 285-289 © TÜBİTAK Research Article doi:10.3906/bot-0812-3 A new taxon of Senecio (Asteraceae) from Turkey: Senecio inops Boiss. & Balansa subsp. karamanicus Hamzaoğlu & Budak Ergin HAMZAOĞLU1,*, Ümit BUDAK1, Ahmet AKSOY2 1Bozok University, Faculty of Science and Arts, Department of Biology, 66200 Yozgat - TURKEY 2Erciyes University, Faculty of Science and Arts, Department of Biology, 38039 Kayseri - TURKEY Received: 17.12.2008 Accepted: 02.06.2009 Abstract: Senecio inops Boiss. & Balansa subsp. karamanicus Hamzaoğlu & Budak (Asteraceae, Senecioneae) is described as a new subspecies from Karaman Province (Inner/South Anatolia). A Latin diagnosis, a taxonomic description, an illustration of the new subspecies, geographical distribution, and some comments on its affinity withSenecio inops Boiss. & Balansa subsp. inops are given. Key words: Asteraceae, Karaman, Senecio, taxonomy, Turkey Türkiye’den Senecio’nun (Asteraceae) yeni bir taksonu: Senecio inops Boiss. & Balansa subsp. karamanicus Hamzaoğlu & Budak Özet: Senecio inops Boiss. & Balansa subsp. karamanicus Hamzaoğlu & Budak (Asteraceae, Senecioneae) Karaman ilinden yeni bir alttür olarak tanımlandı (İç/Güney Anadolu). Yeni alttürün Latince kısa ayrımı, taksonomik betimlemesi, resmi, coğrafik yayılışı ve Senecio inops Boiss. & Balansa subsp. inops ile yakınlığı hakkında bazı yorumlar verildi. Anahtar sözcükler: Asteraceae, Karaman, Senecio, taksonomi, Türkiye Introduction infrageneric classification of Senecio difficult, and Senecioneae is one of the largest tribes of therefore, the evolutionary history of this genus is still Asteraceae, comprising about 150 genera and 3000 poorly known (Jeffrey et al., 1977; Bremer, 1994; species. Senecio L. is one of about 50 plant genera that Vincent, 1996; Mabberley, 1997). contain over 500 species. The extent of the genus The generic and infrageneric concepts of Senecio (about 1500 species) has made attempts at s.l. -

A Revision of Senecio L. (Asteraceae, Senecioneae), in Iran
A REVISION OF SENECIO L. (ASTERACEAE, SENECIONEAE), IN IRAN E. Lotfi, M. Yousofi & M. Assadi Received 20 02 2010. Accepted for publication 12 05 2010 Lotfi, E., Yousofi, M. & Assadi, M. 2010 06 30: A revision of Senecio L. (Asteraceae, Senecioneae), in Iran. -Iran. J. Bot. 16 (1): 96-106. Tehran. The genus Senecio L. is reviewed based on the literature and available herbarium materials. A synopsis and identification key is provided to the 22 accepted taxa. Diversity centers and distribution maps of Senecio species in Iran are presented. Also the checklist of accepted species and synonyms including selected studied specimens are given. Furthermore, Senecio erucifolius subsp. erucifolius which has been collected from Azerbaijan province is reported as a new subspecies to Iran. This subspecies is compared with closely related subspecies S. erucifolius subsp. grandidentatus. Elahe Lotfi (correspondence), Science Faculty, Payame Noor University, Najaf Abad Center, Najaf Abad. – Mehdi Yousefi, Payam Noor University of Isfahan, Department of Biology, P. O. Box 81395-671, Ashrafi Isfahani Blvd., Isfahan, Iran. E-mail: [email protected]. Mostafa Assadi, Department of Botany, Research Institute of Forests and Rangelands, Tehran, Iran. Key words. Senecio, synopsis, Iran, taxonomy, new record, flora. Senecio L. (Asteraceae, Senecioneae) . . !" . #$ % &' ())*$ +, -.%/ .7 + $ +49 ,$ 22 & .7+45 6 3 . .+ 2 % # 1 Senecio 0' A % @ 2. + .+ 5 ?$$ ;>=. < 0' ; .+ 5 - +) % :$ < %#2' FE2 3 + S. erucifolius subsp. erucifolius+ 5 3 %CD + . 5 +B .+ 2 . +,) S. erucifolius subsp. grandidentatus A I + 5 3 + 5 3 ; . H 5 3 ;G% INTRODUCTION Senecio Sect. Quadridentati Boiss. to the genus The first author has been revising the tribe Senecioneae Iranecio. -

General News
Biocontrol News and Information 37(1), 1N–8N www.cabi.org/BNI General News Economic Benefits of Ragwort Biological Control few quantitative data were collected so although it is in New Zealand clear that the flea beetle has had a big impact on rag- wort, the financial benefits could only be speculated Five agents have been introduced to New Zealand on. during a 90-year biological control programme against ragwort (Jacobaea vulgaris). An analysis by To tackle the wetter areas where the flea beetle has Landcare Research, reported in its What’s New in been relatively ineffective, two lepidopteran biocon- Biological of Weeds? newsletter, shows that suc- trol agents that had been successfully introduced to cessful biological control by one of these, the flea Tasmania from Spain – the ragwort plume moth beetle Longitarsus jacobaeae, has returned a ben- (Platyptilia isodactyla) and the ragwort crown borer efit–cost ratio of 14:1 on dairy farms alone over this (Cochylis atricapitana) – were introduced in 2005 period. (see below). As part of the application to release these agents, a survey was conducted on 32 randomly Ragwort was first recorded in New Zealand from selected dairy farms where the flea beetle had pro- Dunedin, South Island in the 1870s, probably vided inadequate control on South Island’s West arriving as a seed contaminant. Like many other Coast to determine the continuing cost of ragwort weeds in the Asteraceae, copious seed production control. Fowler was able to use these data to com- and competitive vegetative growth along with an plete a national benefit–cost analysis to extrapolate ability to thrive in disturbed habitats contributed to what the cost of ragwort control would be for dairy its invasive success. -

Frequent Gain and Loss of Pyrrolizidine Alkaloids in the Evolution of Senecio Section Jacobaea (Asteraceae)
PHYTOCHEMISTRY Phytochemistry 66 (2005) 1285–1295 www.elsevier.com/locate/phytochem Frequent gain and loss of pyrrolizidine alkaloids in the evolution of Senecio section Jacobaea (Asteraceae) Pieter B. Pelser a,b,*, Helene de Vos c, Claudine Theuring d, Till Beuerle d, Klaas Vrieling c, Thomas Hartmann d,* a Department of Botany, Miami University, Oxford, OH 45056, USA b Nationaal Herbarium Nederland, Universiteit Leiden Branch, P.O. Box 9514, 2300 RA Leiden, The Netherlands c Institute of Biology, Leiden University, P.O. Box 9516, 2300 RA Leiden, The Netherlands d Institut fu¨r Pharmazeutische Biologie, Technische Universita¨t Braunschweig, Mendelssohnstrasse 1, D-38106 Braunschweig, Germany Received 18 November 2004; received in revised form 12 January 2005 Available online 17 May 2005 Abstract Pyrrolizidine alkaloids (PAs) of the macrocyclic senecionine type are secondary metabolites characteristic for most species of the genus Senecio (Asteraceae). These PAs are deterrent and toxic to most vertebrates and insects and provide plants with a chemical defense against herbivores. We studied the PA composition of 24 out of 26 species of Senecio section Jacobaea using GC-MS. The PA profiles of eight of these species have not been studied before and additional PAs were identified for most other species that were included in previous studies. With one exception (senecivernine) all 26 PAs identified in sect. Jacobaea can be regarded as derivatives of the biosynthetic backbone structure senecionine. Based on the PA profiles of the species of sect. Jacobaea and the results of pre- vious tracer studies, we constructed two hypothetical biosynthetic scenarios of senecionine diversification. Both scenarios contain two major reactions: the conversion of the necine base moiety retronecine into the otonecine moiety and site-specific epoxidations within the necic acid moiety. -

Guidebook to Invasive Nonnative Plants of the Elwha Watershed Restoration
Guidebook to Invasive Nonnative Plants of the Elwha Watershed Restoration Olympic National Park, Washington Cynthia Lee Riskin A project submitted in partial fulfillment of the requirements for the degree of Master of Environmental Horticulture University of Washington 2013 Committee: Linda Chalker-Scott Kern Ewing Sarah Reichard Joshua Chenoweth Program Authorized to Offer Degree: School of Environmental and Forest Sciences Guidebook to Invasive Nonnative Plants of the Elwha Watershed Restoration Olympic National Park, Washington Cynthia Lee Riskin Master of Environmental Horticulture candidate School of Environmental and Forest Sciences University of Washington, Seattle September 3, 2013 Contents Figures ................................................................................................................................................................. ii Tables ................................................................................................................................................................. vi Acknowledgements ....................................................................................................................................... vii Introduction ....................................................................................................................................................... 1 Bromus tectorum L. (BROTEC) ..................................................................................................................... 19 Cirsium arvense (L.) Scop. (CIRARV) -

The Evolution of Pyrrolizidine Alkaloid Diversity Among and Within Jacobaea Species
Journal of Systematics JSE and Evolution doi: 10.1111/jse.12671 Research Article The evolution of pyrrolizidine alkaloid diversity among and within Jacobaea species Yangan Chen1* , Patrick P. J. Mulder2, Onno Schaap1, Johan Memelink1, Peter G. L. Klinkhamer1, and Klaas Vrieling1 1Institute of Biology, Leiden University, Sylviusweg 72, P. O. Box 9505, Leiden 2300 RA, The Netherlands 2RIKILT‐Wageningen University & Research, Akkermaalsbos 2, P.O. Box 230, Wageningen 6700 AE, The Netherlands *Author for correspondence. E‐mail: [email protected] Received 28 April 2020; Accepted 27 July 2020; Article first published online 8 August 2020 Abstract Plants produce many secondary metabolites showing considerable inter‐ and intraspecific diversity of concentration and composition as a strategy to cope with environmental stresses. The evolution of plant defenses against herbivores and pathogens can be unraveled by understanding the mechanisms underlying chemical diversity. Pyrrolizidine alkaloids are a class of secondary metabolites with high diversity. We performed a qualitative and quantitative analysis of 80 pyrrolizidine alkaloids with liquid chromatography‐tandem mass spectrometry of leaves from 17 Jacobaea species including one to three populations per species with 4–10 individuals per population grown under controlled conditions in a climate chamber. We observed large inter‐ and intraspecific variation in pyrrolizidine alkaloid concentration and composition, which were both species‐specific. Furthermore, we sequenced 11 plastid and three nuclear regions to reconstruct the phylogeny of the 17 Jacobaea species. Ancestral state reconstruction at the species level showed mainly random distributions of individual pyrrolizidine alkaloids. We found little evidence for phylogenetic signals, as nine out of 80 pyrrolizidine alkaloids showed a significant phylogenetic signal for Pagel's λ statistics only, whereas no significance was detected for Blomberg's K measure.