Full Text in Pdf Format
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Metapopulation Model for Whale-Fall Specialists: the Largest Whales Are Essential to Prevent Species Extinctions
THE SEA: THE CURRENT AND FUTURE OCEAN Journal of Marine Research, 77, Supplement, 283–302, 2019 A metapopulation model for whale-fall specialists: The largest whales are essential to prevent species extinctions by Craig R. Smith,1,2 Joe Roman,3 and J. B. Nation4 ABSTRACT The sunken carcasses of great whales (i.e., whale falls) provide an important deep-sea habitat for more than 100 species that may be considered whale-fall specialists. Commercial whaling has reduced the abundance and size of whales, and thus whale-fall habitats, as great whales were hunted and removed from the oceans, often to near extinction. In this article, we use a metapopulation modeling approach to explore the consequences of whaling to the abundance and persistence of whale-fall habitats in the deep sea and to the potential for extinction of whale-fall specialists. Our modeling indicates that the persistence of metapopulations of whale-fall specialists is linearly related to the abundance of whales, and extremely sensitive (to the fourth power) to the mean size of whales. Thus, whaling-induced declines in the mean size of whales are likely to have been as important as declines in whale abundance to extinction pressure on whale-fall specialists. Our modeling also indicates that commercial whaling, even under proposed sustainable yield scenarios, has the potential to yield substantial extinction of whale-fall specialists. The loss of whale-fall habitat is likely to have had the greatest impact on the diversity of whale-fall specialists in areas where whales have been hunted for centuries, allowing extinctions to proceed to completion. -

Role of Cetaceans in Ecosystem Functioning
WORKSHOP REPORT Role of Cetaceans in Ecosystem Functioning: Defining Marine Conservation Policies in the 21st Century 28th International Congress for Conservation Biology Society for Conservation Biology Workshop Report Role of Cetaceans in Ecosystem Functioning: Defining Marine Conservation Policies in the 21st Century 28th International Congress for Conservation Biology Society for Conservation Biology 26 July 2017, Cartagena, Colombia Room Barahona 1, Cartagena Convention Center www.ccc-chile.org www.icb.org.ar www.whales.org www.oceancare.org www.hsi.org csiwhalesalive.org www.nrdc.org www.minrel.gob.cl www.belgium.be For centuries, the great whales (baleen whales and the scientists, but to ecological economists (who ascribe finan- sperm whale) and other cetaceans1 (small whales, dolphins cial values to ecological functions) and to and porpoises) were valued almost exclusively for their oil policymakers concerned with conserving biodiversity. and meat. Widespread commercial hunting reduced great These services confirm what the public, since the early whale numbers by as much as 90 percent, with some ‘Save the Whale’ movement in the 1970s, has always un- populations being hunted to extinction. derstood; cetaceans are special. In recent decades, changing attitudes toward protecting The global implications of the significant contributions of wildlife and the natural world and the growth of ecotourism cetaceans “to ecosystem functioning that are beneficial for provided new cultural and non-extractive economic values the natural environment and people” were first formally for these marine mammals. acknowledged in 2016 when the International Whaling Commission (IWC) adopted a resolution on Cetaceans and Today, whale watching is worth more than $2 billion annu- Their Contributions to Ecosystem Functioning2. -

Deep-Sea Biology
Deep-Sea Biology (OCN430) - Syllabus Fall 2017 Instructors: Jeff Drazen, office MSB605, [email protected], 956-6567 Craig Smith, office MSB617, [email protected], 956-7776 T TH 12:00-1:15 POST708 Syllabus schedule subject to change Course Goals – The deep sea is the largest living space on the planet. Its inhabitants are varied and its communities are often complex, adapted to the particular characteristics of their habitat. This course will cover the major topics in the field, such as bentho-pelagic coupling, depth zonation, energetics, diversity, ecosystem function, adaptations, and the ecology of major habitats. The last portion of the course will deal with anthropogenic threats such as deep-sea fisheries, mining and global climate change. Its goal is to provide you with a basic understanding of what we know (and don’t know) about the biology, ecology and biodiversity of deep-sea ecosystems, the methods used in the field, and it will create a forum for discussion of the major current questions and recent exciting discoveries. Course Structure – After each lecture (or pair of lectures) students will lead a discussion session. The lectures will present the basics of the topics. The discussions will be based on assigned readings (primarily current scientific papers), allowing the class to explore the controversies, implications of recent findings, and highlight future directions for research. Student Learning Outcomes – At the end of this course you will be able to: 1) Describe the co-varying effects of temperature, pressure, oxygen and light levels on the adaptations of deep-sea organisms. 2) Evaluate the influence of variables co-varying with depth on communities, populations, and species. -
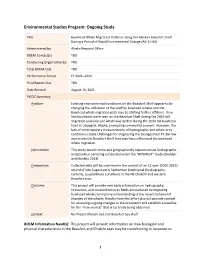
SDP PICOC Template
Environmental Studies Program: Ongoing Study Title Bowhead Whale Migration Patterns along the Alaskan Beaufort Shelf During a Period of Rapid Environmental Change (AK-21-04) Administered by Alaska Regional Office BOEM Contact(s) TBD Conducting Organization(s) TBD Total BOEM Cost TBD Performance Period FY 2021–2024 Final Report Due TBD Date Revised August 19, 2021 PICOC Summary Problem Evolving environmental conditions on the Beaufort Shelf appear to be changing the utilization of the shelf by bowhead whales and the bowhead whale migration path may be shifting farther offshore. Very few bowheads were seen on the Beaufort Shelf during the 2019 fall migration and only one whale was landed during the 2019 fall bowhead hunt at Utqiaġvik, Alaska, prompting community concern. However, the lack of contemporary measurements of hydrographic and whale prey conditions create challenges for diagnosing the changes near Pt. Barrow and across the Beaufort Shelf that may have influenced the bowhead whale migration. Intervention This study would renew and geographically expand annual hydrographic and plankton sampling conducted under the “BOWFEST” study (Shelden and Mocklin 2013). Comparison Collected data will be examined in the context of an 11-year (2005-2015) record of late August-early September biophysical (hydrography, currents, zooplankton) conditions in the NE Chukchi and western Beaufort seas. Outcome This project will provide new basic information on hydrography, circulation, and zooplankton prey fields encountered by migrating bowhead whales to improve understanding of the recent behavioral changes of the whales. Results from this effort also will provide context for assessing ongoing changes to the ecosystem and establish a baseline for the “new normal” that is currently being observed. -

Articles and Detrital Matter
Biogeosciences, 7, 2851–2899, 2010 www.biogeosciences.net/7/2851/2010/ Biogeosciences doi:10.5194/bg-7-2851-2010 © Author(s) 2010. CC Attribution 3.0 License. Deep, diverse and definitely different: unique attributes of the world’s largest ecosystem E. Ramirez-Llodra1, A. Brandt2, R. Danovaro3, B. De Mol4, E. Escobar5, C. R. German6, L. A. Levin7, P. Martinez Arbizu8, L. Menot9, P. Buhl-Mortensen10, B. E. Narayanaswamy11, C. R. Smith12, D. P. Tittensor13, P. A. Tyler14, A. Vanreusel15, and M. Vecchione16 1Institut de Ciencies` del Mar, CSIC. Passeig Mar´ıtim de la Barceloneta 37-49, 08003 Barcelona, Spain 2Biocentrum Grindel and Zoological Museum, Martin-Luther-King-Platz 3, 20146 Hamburg, Germany 3Department of Marine Sciences, Polytechnic University of Marche, Via Brecce Bianche, 60131 Ancona, Italy 4GRC Geociencies` Marines, Parc Cient´ıfic de Barcelona, Universitat de Barcelona, Adolf Florensa 8, 08028 Barcelona, Spain 5Universidad Nacional Autonoma´ de Mexico,´ Instituto de Ciencias del Mar y Limnolog´ıa, A.P. 70-305 Ciudad Universitaria, 04510 Mexico,` Mexico´ 6Woods Hole Oceanographic Institution, MS #24, Woods Hole, MA 02543, USA 7Integrative Oceanography Division, Scripps Institution of Oceanography, La Jolla, CA 92093-0218, USA 8Deutsches Zentrum fur¨ Marine Biodiversitatsforschung,¨ Sudstrand¨ 44, 26382 Wilhelmshaven, Germany 9Ifremer Brest, DEEP/LEP, BP 70, 29280 Plouzane, France 10Institute of Marine Research, P.O. Box 1870, Nordnes, 5817 Bergen, Norway 11Scottish Association for Marine Science, Scottish Marine Institute, Oban, -

Whales, Wood and Kelp Islands in the Deep-Sea
ANGELO FRAGA BERNARDINO Whales, wood and kelp islands in the deep-sea: ecological succession and species overlap with other chemosynthetic habitats in the Californian continental slope (NE Pacific) Tese apresentada ao Instituto Oceanográfico da Universidade de São Paulo, como parte dos requisitos para obtenção do título de Doutor em Ciências, área de Oceanografia Biológica. Orientador: Prof. Dr. Paulo Yukio Gomes Sumida Co-orientador: Prof. Dr. Craig R. Smith São Paulo 2009 Universidade de São Paulo Instituto Oceanográfico Whales, wood and kelp islands in the deep-sea: ecological succession and species overlap with other chemosynthetic habitats in the Californian continental slope (NE Pacific) Angelo Fraga Bernardino Tese apresentada ao Instituto Oceanográfico da Universidade de São Paulo, como parte dos requisitos para obtenção do título de Doutor em Ciências, área de Oceanografia Biológica. Julgada em ___/___/_____ Prof.(a) Dr.(a) Conceito Prof.(a) Dr.(a) Conceito Prof.(a) Dr.(a) Conceito Prof.(a) Dr.(a) Conceito Prof.(a) Dr.(a) Conceito i INDEX List of Tables ……………………………………………………………….………… ii List of Figures ………………………………………………………………..………. iii Abstract ………………………………………………………………..……………… v Resumo ………………………………………………………………..……………… vi Chapter 1. Introduction ………………………………………………….…………….. 1 1.1. References ..………………………..….………………………………..…........ 6 Chapter 2. Macrofaunal succession in sediments around kelp and wood falls in the 9 deep NE Pacific and community overlap with other reducing habitats 2.1. Introduction ……………………………………………………………………. 10 2.2. Materials and methods ……………...........…………………………………….. 12 2.3. Results ………………………............................................................................. 19 2.4. Discussion …………………………….……………………………………….. 38 2.5. Conclusions …………….……………………………………………………… 46 2.6. References ……….…………………………………………………………….. 46 2.7. Supplementary material …………………………….…………………………. 55 Chapter 3. Infaunal community structure and succession during the sulfophilic stage 57 of a whale carcass in the deep NE Pacific 3.1. -

Ecology of Whale Falls at the Deep-Sea Floor
Oceanography and Marine Biology: an Annual Review 2003, 41, 311–354 © R.N. Gibson and R.J.A. Atkinson, Editors Taylor & Francis ECOLOGY OF WHALE FALLS AT THE DEEP-SEA FLOOR CRAIG R. SMITH1 & AMY R. BACO1,2 1Department of Oceanography, University of Hawaii at Manoa, 1000 Pope Road, Honolulu, HI, 96822, USA e-mail: [email protected] 2present address: Biology Department, Woods Hole Oceanographic Institution, Woods Hole, MA 02543, USA e-mail: [email protected] Abstract The falls of large whales (30–160t adult body weight) yield massive pulses of labile organic matter to the deep-sea floor. While scientists have long speculated on the ecological roles of such concentrated food inputs, observations have accumulated since the 1850s to suggest that deep-sea whale falls support a widespread, characteristic fauna. Interest in whale- fall ecology heightened with the discovery in 1989 of a chemoautotrophic assemblage on a whale skeleton in the northeast Pacific; related communities were soon reported from whale falls in other bathyal and abyssal Pacific and Atlantic sites, and from 30mya (million years ago) in the northeast Pacific fossil record. Recent time-series studies of natural and implanted deep- sea whale falls off California, USA indicate that bathyal carcasses pass through at least three successional stages: (1) a mobile-scavenger stage lasting months to years, during which aggregations of sleeper sharks, hagfish, rat-tails and invertebrate scavengers remove whale soft tissue at high rates (40–60kgdϪ1); (2) an enrichment opportunist stage (duration of months to years) during which organi- cally enriched sediments and exposed bones are colonised by dense assemblages (up to 40000mϪ2) of opportunistic polychaetes and crustaceans; (3) a sulphophilic (“or sulphur-loving”) stage lasting for decades, during which a large, species-rich, trophically complex assemblage lives on the skeleton as it emits sul- phide from anaerobic breakdown of bone lipids; this stage includes a chemoau- totrophic component deriving nutrition from sulphur-oxidising bacteria. -

Whale-Fall Ecosystems: Recent Insights Into Ecology, Paleoecology, and Evolution
MA07CH24-Smith ARI 28 October 2014 12:32 Whale-Fall Ecosystems: Recent Insights into Ecology, Paleoecology, and Evolution Craig R. Smith,1 Adrian G. Glover,2 Tina Treude,3 Nicholas D. Higgs,4 and Diva J. Amon1 1Department of Oceanography, University of Hawaii, Honolulu, Hawaii 96822; email: [email protected], [email protected] 2Department of Life Sciences, Natural History Museum, SW7 5BD London, United Kingdom; email: [email protected] 3GEOMAR Helmholtz Centre for Ocean Research Kiel, 24148 Kiel, Germany; email: [email protected] 4Marine Institute, Plymouth University, PL4 8AA Plymouth, United Kingdom; email: [email protected] Annu. Rev. Mar. Sci. 2015. 7:571–96 Keywords First published online as a Review in Advance on ecological succession, chemosynthesis, speciation, vent/seep faunas, September 10, 2014 Osedax, sulfate reduction The Annual Review of Marine Science is online at marine.annualreviews.org Abstract This article’s doi: Whale falls produce remarkable organic- and sulfide-rich habitat islands at 10.1146/annurev-marine-010213-135144 Access provided by 168.105.82.76 on 01/12/15. For personal use only. the seafloor. The past decade has seen a dramatic increase in studies of mod- Copyright c 2015 by Annual Reviews. ern and fossil whale remains, yielding exciting new insights into whale-fall All rights reserved Annu. Rev. Marine. Sci. 2015.7:571-596. Downloaded from www.annualreviews.org ecosystems. Giant body sizes and especially high bone-lipid content allow great-whale carcasses to support a sequence of heterotrophic and chemosyn- thetic microbial assemblages in the energy-poor deep sea. -

Hagfish Ampharetid Worms Giant Isopod Osedax Worm Sea Pig
Grooved Tanner Crab Rattail Fish Squat Lobster Sixgill Shark Scientific Name: Chionoecetes tanneri Scientific Name:....... Coryphaenoides Scientific Name: .........Munidopsis spp. Scientific Name:... Hexanchus griseus acrolepis Average Size: .............0.08-6.3 inches Size: .................. Up to 16 feet in length Size: .........................................1-3 feet Squat lobsters have short, flattened Depth Range: ...............174-6,378 feet Depth Range: .............. 656-3,280 feet Depth Range: .......... 650 ft - 2.5 miles bodies and long antennae that are used to locate objects and maintain Life span ....................... up to 70 years One of three species sold as snow distance from other lobsters. They Sixgill sharks can be found around crab for consumption, grooved typically eat small worms or crusta- the world. These reclusive creatures Tanner crabs have a deep groove Rattail fish, or grenadiers, are curious ceans or scavenge on dead organ- are usually found in very deep water, running down the center of their fish that have adapted to thrive in the isms. Squat lobsters have long claws making them hard to study. These shells. These crabs have four pairs of dark ocean. They have large eyes that can be up to twice as long as sharks feed or scavenge on fish, long thin legs and one pair of shorter that can detect bioluminescent organ- their bodies. crustaceans, rays, and sometimes legs equipped with pincers. isms, and sensory structures on their seals and other sharks. heads to help sense food sources. FUN FACT: Squat lobsters look like FUN FACT: Chionoecetes means lobsters, but they are actually more FUN FACT: As their name suggests, snow (chio) inhabitant (ioketes), FUN FACT: Some rattails use their closely related to hermit crabs. -

Program and Abstract Book
11th International Deep-Sea Biology Symposium NATIONAL OCEANOGRAPHY cENTRE, Southampton, UK 9 - 14 July 2006 BOOK OF ABSTRACTS Compiled by: Sven Thatje Paul Tyler Pam talbot tammy horton Lis Maclaren Sarah Murty Nina Rothe david billett 11th International Deep-Sea Biology Symposium National Oceanography Centre, Southampton Southampton Solent University Conference Centre Southampton UK 9 – 14 July 2006 Symposium Organising Committee • Professor Paul Tyler (Chair), NOC DEEPSEAS Group, UK. • Mrs Pam Talbot (Secretary), George Deacon Division, NOC, UK. • Dr David Billett, NOC DEEPSEAS Group, UK. • Dr Sven Thatje, NOC DEEPSEAS Group, UK. • Professor Monty Priede, OceanLab, University of Aberdeen, UK. • Dr Gordon Paterson, The Natural History Museum, London, UK. • Professor George Wolff, University of Liverpool, UK. • Dr Kerry Howell, Joint Nature Conservation Committee, UK. • Dr Alex Rogers, British Antarctic Survey, Cambridge, UK. • Dr Eva Ramirez Llodra, NOC DEEPSEAS/CSIC Barcelona, Spain. • Dr Phil Bagley, OceanLab, University of Aberdeen, UK. • Dr Maria Baker, NOC DEEPSEAS Group, UK. • Dr Brian Bett, NOC DEEPSEAS Group, UK. • Dr Jon Copley, NOC DEEPSEAS Group, UK. • Dr Adrian Glover, The Natural History Museum, London, UK. • Professor Andrew Gooday, NOC DEEPSEAS Group, UK. • Dr Lawrence Hawkins, NOC DEEPSEAS Group, UK. • Dr Tammy Horton, NOC DEEPSEAS Group, UK. • Dr Ian Hudson, NOC DEEPSEAS Group, UK. • Dr Alan Hughes, NOC DEEPSEAS Group, UK. • Dr Bhavani Narayanaswamy, Scottish Association for Marine Science, Oban, UK. • Dr Martin Sheader, NOC DEEPSEAS Group, UK. • Miss Michelle Sterckx, Southampton Solent University Conference Centre, UK. • Dr Ben Wigham, OceanLab, University of Aberdeen, UK. • Supported by the DEEPSEAS post-graduate/doctorate team: Lis Maclaren, Sarah Murty, Nina Rothe, Tania Smith, John Dinley, Chris Hauton, Jon Copley, Hannah Flint, Abigail Pattenden, Emily Dolan, Teresa Madurell, Teresa Amaro, Janne Kaariainen, Daniel Jones, Kate Larkin, Eulogio Soto. -

The Early Pleistocene Whale-Fall Community of Bargiano (Umbria, Central Italy): Paleoecological Insights from Benthic Foraminifera and Brachyuran Crabs
Palaeontologia Electronica palaeo-electronica.org The early Pleistocene whale-fall community of Bargiano (Umbria, Central Italy): Paleoecological insights from benthic foraminifera and brachyuran crabs Angela Baldanza, Roberto Bizzarri, Federico Famiani, Alessandro Garassino, Giovanni Pasini, Marco Cherin, and Francesco Rosatini ABSTRACT New insights into communities of benthic foraminifera and decapods, associated with whale-fall events (WFE) in a relatively shallow sea environment, are reported here for the first time from the early Pleistocene of Bargiano (southwestern Umbria, Italy). The inferred paleodepth of these WFEs is not greater than 100−150 m and, on the basis of more general stratigraphic data, took place over an estimated period of about 50,000 years. The foraminifera assemblages associated with these WFEs are low in number of planktonic and benthic taxa, and six benthic species dominate: the shallow infaunal species Bigenerina nodosaria, Bannerella gibbosa, Marginulinopsis costata, and Vaginulina cf. V. striatissima, along with the epifaunal species Lenticulina calcar and Siphotextularia concava. Because these opportunistic species respond to short- term favorable conditions by increasing in number and maintaining stable populations, the presence of high numbers of individuals of these species in association with three recognized WFEs provides evidence that a nutrient-rich environment favored their pro- liferation. The occurrence of previously unreported benthic foraminifera taxa (across the three WFEs), along with the presence of the crab species Albaidaplax ispalensis (Goneplacidae) and Chlinocephalus demissifrons (Euryplacidae) (in at least one WFE), offer new insights into shallow sea whale-fall fossil communities. Angela Baldanza (corresponding author), Department of Physics and Geology, University of Perugia, Via A. Pascoli ‒ I-06123 Perugia, Italy, [email protected] Roberto Bizzarri, Department of Physics and Geology, University of Perugia, Via A. -

Meiofauna Community Composition at Three Whale-Fall Sites In
MEIOFAUNA COMMUNITY COMPOSITION AT THREE WHALE-FALL SITES IN MONTEREY BAY, CALIFORNIA ____________ A Thesis Presented to the Faculty of the Moss Landing Marine Laboratories California State University Monterey Bay ____________ In Partial Fulfillment of the Requirements for the Degree Master of Science in Marine Science ____________ by Gillian Louise Rhett Summer 2014 Copyright © 2014 by Gillian L. Rhett All Rights Reserved ABSTRACT There are localized deep-seafloor habitats where there is a much greater input of nutrients than most of the seafloor. These include hydrothermal vents, cold seeps, and sunken whale skeletons called whale-falls. Meiofauna, a taxonomically diverse group of microscopic invertebrates and single-celled eukaryotes, have been studied in many habitats, though there have been no published studies on meiofauna at whale-falls. The purpose of this study was to test whether the increased energy resources at a whale-fall affected the meiofauna community. To test this hypothesis, I characterized the community of meiofauna living under and around whale-fall at three locations in the Monterey Bay, in terms of biomass (µg carbon per cm2) and diversity. Nematodes were the most abundant organism, although annelids accounted for the greatest share of biomass in some samples due to their larger body size. More meiofaunal organisms were found near the carcass than far from it and the greatest meiofaunal biomass occurred three to seven meters from the carcass. The greater biomass nearer the bones was probably due to nutrient enrichment from the whale. The lesser numbers under the carcass compared with 3 – 7 m away may be due to toxic chemical gradients in the sediment around the bones, grazing by larger organisms living near the bones, or competitive dominance.