Materials for High Vacuum Technology: an Overview
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nguyen Quoc Tuan Effects of Substituting Ytterbium for Scandium
Universidade do Minho Escola de Engenharia Nguyen Quoc Tuan Effects of substituting ytterbium for scandium on the microstructure and properties of Al-Sc and Al-Mg-Sc alloys Effects of substituting ytterbium for scandium on the microstructure and properties of Al-Sc and Al-Mg-Sc alloys Nguyen Quoc Tuan Outubro de 2014 UMinho | 2014 Universidade do Minho Escola de Engenharia Nguyen Quoc Tuan Effects of substituting ytterbium for scandium on the microstructure and properties of Al-Sc and Al-Mg-Sc alloys Tese de Doutoramento Programa Doutoral em Engenharia de Materiais Trabalho efectuado sob a orientação da Professora Ana Maria Pires Pinto e co-orientação do Professor Luís Augusto Marques Sousa Rocha Outubro de 2014 STATEMENT OF INTEGRITY I hereby declare having conducted my thesis with integrity. I confirm that I have not used plagiarism or any form of falsification of results in the process of the thesis elaboration. I further declare that I have fully acknowledged the Code of Ethical Conduct of the University of Minho. University of Minho, Guimarães, 29th October 2014 Full name: Nguyen Quoc Tuan Signature: _________________________________________________________________ Acknowledgments This research have been carried out during my Ph.D program at Centre for Mechanical and Materials Technologies (CT2M), Department of Mechanical Engineering, University of Minho, Guimaraes, Portugal. There are many people who I would like to thank for their assistance in this thesis research. First and foremost, I would like to express my sincere gratitude and warm regards to my advisors Prof. Ana Maria Pinto and Prof. Luis Rocha for giving me opportunity to complete the Ph.D degree at their laboratory. -

Connor Final Highlighted
Preliminary Development of a PPAM Actuated Pediatric Prosthetic Ankle Connor McNamara-Spackman A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science (Bioengineering) at the University of Otago, Dunedin, New Zealand. February 2021 Abstract The purpose of this research was to develop a preliminary design of a powered pediatric prosthetic ankle. Previous research identified the health risk of improper gait cycle and the lack of powered prosthetic ankle options for children. Costs for powered prosthetic ankles are too high (upwards of $5000 NZD), the sizes are too large and the weight is too significant for a child to benefit from. Current technologies for ankle joint actuation and materials for the prosthetic structure were evaluated and a conclusion of utilizing PPAMs was chosen due to their ability to generate the required 300 N of contraction force. CAD was used to model the structure of a prosthetic ankle and evaluate the FOS of the different material combinations while under static loading and fatigue simulations. HDPE and UHMWPE failed to withstand the simulations, while the aluminium alloy and stainless steel showed minimal faults from the simulations. MatLab was used to simulate the desired PPAM dimensions of 100 mm to determine the contraction force and contraction percentage that can be generated by the PPAM. The smallest PPAM found in research was 110 mm and showed promising results from their mathematical modeling. The overall height of the prosthetic was no greater than 110 mm and the membrane length of the PPAM was no greater than 100 mm, while successfully producing more than 300 N during contraction. -

Trabalho De Diplomação Estudo Da Corrosão
UNIVERSIDADE FEDERAL DO RIO GRANDE DO SUL ESCOLA DE ENGENHARIA ENGENHARIA DE MATERIAIS ENG 02298 - TRABALHO DE DIPLOMAÇÃO ESTUDO DA CORROSÃO LOCALIZADA DAS LIGAS AA 2024 E AA 2198 ENDURECIDAS POR PRECIPITAÇÃO Jéssica Salles Pinheiro 205994 Orientador: Prof. Dr. Luís Frederico Pinheiro Dick Co-orientador: Pedro Atz Dick Dezembro de 2015 1 AGRADECIMENTOS Pelo apoio que recebi direta ou indiretamente para a concretização do presente trabalho sou grata às seguintes pessoas: Ao meu orientador, Luís Frederico Pinheiro Dick, pelos conhecimentos passados e pela confiança em minha independência na execução do trabalho. Ao meu co-orientador, Pedro Atz Dick, pelo auxílio imprescindível em cada etapa do trabalho e por não poupar esforços para repassar seu conhecimento desde o início de minha experiência no laboratório. Ao mestrando Lucas Travi, pelo acesso e ajuda no uso do microdurômetro do LdTM. Aos meus pais, Tânia e Diógenes, que me proporcionaram as melhores oportunidades possíveis até hoje e incentivaram minha vida de estudante desde o início, além de me apoiarem financeira e emocionalmente. À minha irmã, Francielli, que sempre me apoiou em todas as decisões que tomei, lembrando como tenho sorte por ter sua amizade incondicional em minha vida. À minha namorada, Priscila, pela paciência nos dias difíceis e por estar sempre ao meu lado me motivando. À minha amiga Luísa, por ter prontamente me ajudado nas análises de microscopia de varredura, dispondo de mais de um dia para isso, além de ser meu porto seguro emocional em todos os momentos. À minha amiga Alana, por sua amizade valiosa e pela sincera preocupação que demonstrou desde o início do trabalho. -

Parshwamani Metals
+91-8048554624 Parshwamani Metals https://www.indiamart.com/parshwamanimetals/ Parshwamani Metals is one of the leading manufacturers, supplier and traders of Industrial Metal Tube, Beryllium Product, Shim Sheet, SS Round And Square Bar, Aluminium Products, Aluminum Bronze Products etc. About Us Parshwamani Metals was established in the year 2015 as a professionally managed Manufacturer, Trader and Wholesaler specialized in providing premium grade Copper and Brass Metals Products. Today, we endeavor to revolutionize the industry by fabricating a wide gamut of quality products, which includes Brass Products, Copper Products and Copper Alloy. Our claim to success is hallmarked by the offered quality products that gained us huge recognizance for its high strength, wear and tear resistance, accurate dimensions, flexibility and durable finish. Our products find their wide applications in architectural fittings, hardware and telecommunication. Owing to swift delivery schedules, easy payment modes and overt business practices, we have been successful in earning huge client base. We deal in Jindal Brand. Our efforts are determined with the objective of industrial leadership that equips our team members to manufacture customized products. And, to achieve this, we have developed modernized R&D centers and cutting edge manufacturing facilities. Furthermore, the facility is divided into various functional units like procurement, engineering, production, research & development, quality-testing, warehousing & packaging etc. Our organization is backed -
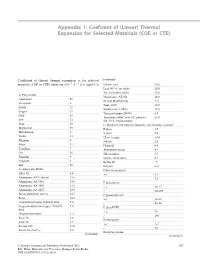
Linear) Thermal Expansion for Selected Materials (COE Or CTE
Appendix 1: Coefcient of (Linear) Thermal Expansion for Selected Materials (COE or CTE) Coefficient of (linear) thermal expansion, α, for selected (continued) − − materials (COE or CTE) (units are ×10 6 °C 1 (i.e. ppm/°C)) Indium–lead 33.0 Lead (95 %) tin solder 28.0 Tin–lead solder 60/40 25.0 A. Pure metals Magnesium, AZ31B 26.0 Aluminium 25 Ni-clad Molybdenum 5–6 Chromium 6 Steel, 1020 12.0 Cobalt 12 Stainless steel (18-8) 17.0 Copper 17 Tungsten/copper (90/10) 6.5 Gold 14 Aluminium MMC with SiC particles 6–14 Iron 12 (80–50 % reinforcement) Lead 29 C. Insulators and substrate materials (for electronic systems)a Magnesium 25 E glass 5.5 Molybdenum 5 S glass 2.6 Nickel 13 Glass–ceramic >3.0 Platinum 9 Silicon 2.6 Silver 19 Diamond 0.9 Tantalum 7 Aluminium nitride 4.5 Tin 20 Silicon nitride 3.7 Titanium 9 Quartz, fused silica 0.5 Tungsten 5 Kevlar 49 –5 Zinc 35 Beryllia 6–9 B. Alloys and MMCs Cubic boron nitride Alloy 42 4.4 x–y 3.7 Aluminium (40 % silicon) 13.5 z 7.2 Aluminium, AA 6061 23.6 E glass/epoxy Aluminium, AA 3003 23.2 x–y 14–17 Aluminium, AA 2017 22.9 z 80–280 Boron aluminium (20 %) 12.7 E glass/polyimide Brass 18.0 x–y 12–16 Copper/invar/copper 20/60/20 thick 5.8 z 40–80 Copper/molybdenum/copper 20/60/20 7.0 E glass/PTFE thick x–y 24 Graphite/aluminium 4–6 z 260 Invar 36 1.6 Kevlar/epoxy Invar 42 4.5 x–y 5–7 Inconel 600 13.0 z 70 Kovar (Fe–Ni–Co) 5.0 (continued) Kevlar/polyimide (continued) © Springer International Publishing Switzerland 2016 557 B.D. -

924 Iaea-Sm-310/ 69P
924 IAEA-SM-310/ 69P RESULTS FROM POST-MORTEM TESTS WITH MATERIAL FROM THE OLD CORE-BOX OF THE HIGH FLUX REACTOR (HFR) AT PETTEN M.I. de Vries Netherlands Energy Research Foundation, ECN, Eetten, The Netherlands M.R.. Cundy Joint Research Centre, Patten, The Netherlands 925 RESULTS FROM POST-MORTEM TESTS WITH MATERIAL FROM THE OLD CORE-BOX OF THE HIGH FLUX REACTOR (HFR) AT PETTEN ABSTRACT Results are reported from hardness measurements, tensile tests and fracture mechanics experiments (fatigue crack growth and fracture toughness) on 5154 aluminium specimens, fabricated from remnants of the old HFR core box. The specimen material was exposed to a maximum thermal neutron fluence of 7.5 * 1026n/m2(E < 0.4eV). Test results for this fluence (ratio of the thermal to fast neutron flux density is 1.17) are: hardness 63HR15N, 0.2 - yield strength 525 MPa and total elongation 2.2% strain. Material which was exposed to a lower thermal fluence of 5.6 * 10 n/m , but with a thermal to fast neutron ratio of about 4, shows more radiation hardening : 67HR15N, 0.2 - yield strength 580 MPa and 1.5% total elongation. -5 -3 Fatigue crack growth rates range from 5 * 10 mm/cycle to 10 mm/cycle for AK ranging from 8 to 20 MPa)|m. The most highly exposed (7.5 * 10 n/m ) material shows accelerated fatigue crack growth due to unstable crack extension at AK of about 15 Mpa^m. The lowermost meaningful measure of plane strain fracture toughness is 18 MPa^m. Except for the fracture toughness which is a factor of about 3 higher the results show reasonable agreement with the expected mechanical properties estimated in the "safe end-of-life" assessment of the old HFR vessel. -

Tribological Behaviours Influenced by Surface Coatings and Morphology
University of Windsor Scholarship at UWindsor Electronic Theses and Dissertations Theses, Dissertations, and Major Papers 7-11-2015 Tribological Behaviours Influenced yb Surface Coatings and Morphology Guang Wang University of Windsor Follow this and additional works at: https://scholar.uwindsor.ca/etd Recommended Citation Wang, Guang, "Tribological Behaviours Influenced yb Surface Coatings and Morphology" (2015). Electronic Theses and Dissertations. 5305. https://scholar.uwindsor.ca/etd/5305 This online database contains the full-text of PhD dissertations and Masters’ theses of University of Windsor students from 1954 forward. These documents are made available for personal study and research purposes only, in accordance with the Canadian Copyright Act and the Creative Commons license—CC BY-NC-ND (Attribution, Non-Commercial, No Derivative Works). Under this license, works must always be attributed to the copyright holder (original author), cannot be used for any commercial purposes, and may not be altered. Any other use would require the permission of the copyright holder. Students may inquire about withdrawing their dissertation and/or thesis from this database. For additional inquiries, please contact the repository administrator via email ([email protected]) or by telephone at 519-253-3000ext. 3208. Tribological Behaviours Influenced by Surface Coatings and Morphology By Guang Wang A thesis Submitted to the Faculty of Graduate Studies through the Department of Mechanical, Automotive & Materials Engineering in Partial Fulfillment of the Requirements for the Degree of Master of Applied Science at the University of Windsor Windsor, Ontario, Canada 2015 ©2015 Guang Wang Tribological Behaviours Influenced by Surface Coatings and Morphology By Guang Wang APPROVED BY: Dr. -

Abstracts from the Scientific and Technical Press Titles And
December, ig4j Abstracts from the Scientific and Technical Press " (No. 117. October, 1943) AND Titles and References of Articles and Papers Selected from Publications (Reviewed by R.T.P.3) TOGETHER WITH List of Selected Translations (No. 63)' London : "THE ROYAL AERONAUTICAL SOCIETY" with which is incorporated "The Institution of Aeronautical Engineers" 4, Hamilton Place, W.I Telephone: Grosvenor 3515 (3 lines) ABSTRACTS FROM THE SCIENTIFIC AND TECHNICAL PRESS. Issued by the Directorate's of Scientific Research and Technical Development, Ministry of Air craft Production. (Prepared by R.T.P.3.) No. 117. OCTOBER, 1943. Notices and abstracts from the Scientific and Technical Press are prepared primarily for_ the information of Scientific and Technical Staffs. Particular attention is paid to the work carried out in foreign countries, on the assumption that the more accessible British work (for example that published by the Aeronautical Research Committee^ is already known to these Staffs. Requests from scientific and technical staffs for further information of transla tions should be addressed to R.T,P.3, Ministry of Aircraft Production, and not to the Royal Aeronautical Society. Only a limited number of the articles quoted from foreign journals are trans lated and usually only the original can be supplied on loan. If, however, translation is required, application should be made in writing to R.T.P.3, the requests being considered in accordance with existing facilities. ' NOTE.—As far as possible, the country of origin quoted in the items refers to the original source. The Effect of Nitrogen on the Properties of Certain Austenitic Valve Steels. -

Friction Stir Welding of Dissimilar Materials Between Aluminium Alloys and Copper - an Overview
Proceedings of the World Congress on Engineering 2013 Vol III, WCE 2013, July 3 - 5, 2013, London, U.K. Friction Stir Welding of Dissimilar Materials between Aluminium Alloys and Copper - An Overview Mukuna P. Mubiayi. Member, IAENG and Esther T. Akinlabi, Member, IAENG flow in the material, forming a solid state weld. Abstract—Friction Stir Welding (FSW) is a solid state welding process used for welding similar and dissimilar materials. The process is widely used because it produces sound welds and does not have common problems such as solidification and liquefaction cracking associated with the fusion welding techniques. The FSW of Aluminium and its alloys has been commercialised; and recent interest is focused on joining dissimilar materials. However, in order to commercialise the process, research studies are required to characterise and establish process windows. In particular, FSW has inspired researchers to attempt joining dissimilar materials such as aluminium to copper which differ in properties and sound welds with none or limited intermetallic compounds has been produced. In this paper, we review the current research state of FSW between aluminium and copper with a focus on the resulting weld microstructure, mechanical testing and the tools employed to produce the welds and also an insight into future research in this field of study. Fig.1. Schematic diagram of the Friction Stir Welding process [2] Keywords: Aluminium, copper, dissimilar materials, intermetallic compounds, microstructure. It was realised in the development of the FSW process that the tool design is critical in producing sound welds [3]. A basic and conventional design for a FSW tool is shown in I. -
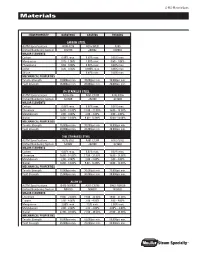
Materialspec Materials
S-MS-MaterialSpec Materials Item/ProPerty Barstock castIng ForgIng carBon steel ASTM Specifications A108-1215 A216-WCB A105 Unified Numbering System G12150 J03002 K03504 maJor elements Carbon 0.09% max. 0.30% max. 0.35% max. Manganese 0.75 - 1.05% 1.00% max. 0.60 - 1.05% Phosphorus 0.04 - 0.09% 0.04% max. 0.04% max. Sulfur 0.26 - 0.35% 0.045% max. 0.05% max. Silicon - 0.60% max. 0.35% max. mecHanIcal ProPertIes Tensile Strength 70,000psi min. 70,000psi min. 70,000psi min. Yield Strength 36,000psi min. 36,000psi min. 36,000psi min. 316 staInless steel ASTM Specifications A276-316 A351-CF8M A182-F316 Unified Numbering System S31600 J92900 S31600 maJor elements Carbon 0.08% max. 0.08% max. 0.08% max. Chromium 16.00 - 18.00% 18.00 - 21.00% 16.00 - 18.00% Molybdenum 2.00 - 3.00% 2.00 - 3.00% 2.00 - 3.00% Nickel 10.00 - 14.00% 9.00 - 13.00% 10.00 -14.00% mecHanIcal ProPertIes Tensile Strength 75,000psi min. 70,000psi min. 75,000psi min. Yield Strength 30,000psi min. 30,000psi min. 30,000psi min. 316l staInless steel ASTM Specifications A276-316L A351-CF3M A182-F316L Unified Numbering System S31603 J92800 S31603 maJor elements Carbon 0.03% max. 0.03% max. 0.03% max. Chromium 16.00 - 18.00% 17.00 - 21.00% 16.00 - 18.00% Molybdenum 2.00 - 3.00% 2.00 - 3.00% 2.00 - 3.00% Nickel 10.00 - 14.00% 9.00 - 13.00% 10.00 - 15.00% mecHanIcal ProPertIes Tensile Strength 70,000psi min. -

Machining of Aluminum and Aluminum Alloys / 763
ASM Handbook, Volume 16: Machining Copyright © 1989 ASM International® ASM Handbook Committee, p 761-804 All rights reserved. DOI: 10.1361/asmhba0002184 www.asminternational.org MachJning of Aluminum and AlumJnum Alloys ALUMINUM ALLOYS can be ma- -r.. _ . lul Tools with small rake angles can normally chined rapidly and economically. Because be used with little danger of burring the part ," ,' ,,'7.,','_ ' , '~: £,~ " ~ ! f / "' " of their complex metallurgical structure, or of developing buildup on the cutting their machining characteristics are superior ,, A edges of tools. Alloys having silicon as the to those of pure aluminum. major alloying element require tools with The microconstituents present in alumi- larger rake angles, and they are more eco- num alloys have important effects on ma- nomically machined at lower speeds and chining characteristics. Nonabrasive con- feeds. stituents have a beneficial effect, and ,o IIR Wrought Alloys. Most wrought alumi- insoluble abrasive constituents exert a det- num alloys have excellent machining char- rimental effect on tool life and surface qual- acteristics; several are well suited to multi- ity. Constituents that are insoluble but soft B pie-operation machining. A thorough and nonabrasive are beneficial because they e,,{' , understanding of tool designs and machin- assist in chip breakage; such constituents s,~ ,.t ing practices is essential for full utilization are purposely added in formulating high- of the free-machining qualities of aluminum strength free-cutting alloys for processing in alloys. high-speed automatic bar and chucking ma- Strain-hardenable alloys (including chines. " ~ ~p /"~ commercially pure aluminum) contain no In general, the softer ailoys~and, to a alloying elements that would render them lesser extent, some of the harder al- c • o c hardenable by solution heat treatment and ,p loys--are likely to form a built-up edge on precipitation, but they can be strengthened the cutting lip of the tool. -

THE VACUUM CHAMBERIN the INTERACTION REGIÓN of PARTIÓLE COLLIDERS: a HISTORICAL STUDY and DEVELOPMENTS IMPLEMENTED in the Lhcb EXPERIMENT at CERN
Departamento de Física Aplicada a la Ingeniería Industrial Escuela Técnica Superior de Ingenieros Industriales THE VACUUM CHAMBERIN THE INTERACTION REGIÓN OF PARTIÓLE COLLIDERS: A HISTORICAL STUDY AND DEVELOPMENTS IMPLEMENTED IN THE LHCb EXPERIMENT AT CERN Autor: Juan Ramón Klnaster Refolio Ingeniero Industrial por la E.T.S.I. Industriales Universidad Politécnica de Madrid Directores: Raymond J.M. Veness Ph; D. Mechanics of Materials and Plasticity University of Leicester (England) Linarejos Gámez Mejías Doctor Ingeniero Industrial por la E.T.S.I.I. Universidad Politécnica de Madrid 2004 Whatever you dream, you can do, begin it! Boldness has power, magic and genius in it Goethe Homo sum: humani nihil a me alienum puto (Je suis homme, et rien de ce que est humain ne m'est étraxiger) Terence Loving softly and deeply... Elsje Tout proche d'étre un Boudha paresseusement réve le vieux pin Issa En nuestra cabeza, en nuestro pecho es donde están los circos en que, vestidos con los disfraces del tiempo, se enfrentan la Libertad y el Destino Jünger This Thesis has been possible thanks to the support of many people that duñng last 15 months have helped me in different ways. I would like to thank my co- lleagues R. Aehy, P. Bryant, B. Calcagno, G. Corii, A. Gerardin, G. Foffano, M. Goossens, C. Hauvüler, H. Kos, J. Kruzelecki, P. Lutkiewicz, T. Nakada, A. Rossi, J.A. Rubio, B. Szybinski, D. Tristram, B. Ver- solatto, L. Vos and W. Witzeling for their contribu- tions in different moments. Neither would I have ever managed to finish it without those moments of peace shared with mes fréres d'Independance et Verité á VOr :.