Benton2009chap04.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Morrison Formation 37 Cretaceous System 48 Cloverly Formation 48 Sykes Mountain Formation 51 Thermopolis Shale 55 Mowry Shale 56
THE STRUCTURAL AND STRATIGRAPHIC FRAMEWORK OF THE WARM SPRINGS RANCH AREA, HOT SPRINGS COUNTY, WYOMING By CHRISTOPHER JAY CARSON Bachelor of Science Oklahoma State University 1998 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE July, 2000 THE STRUCTURAL AND STRATIGRAPHIC FRAMEWORK OF THE WARM SPRINGS RANCH AREA, HOT SPRINGS COUNTY, WYOMING Thesis Approved: Thesis Advisor ~~L. ... ~. ----'-"'-....D~e~e:.-g-e----- II ACKNOWLEDGEMENTS I wish to express appreciation to my advisor Dr. Arthur Cleaves for providing me with the opportunity to compile this thesis, and his help carrying out the fieldwork portion of the thesis. My sincere appreciation is extended to my advisory committee members: Dr. Stan Paxton, Dr. Gary Stewart, and Mr. David Schmude. I wish to thank Mr. Schmude especially for the great deal of personal effort he put forth toward the completion of this thesis. His efforts included financial, and time contributions, along with invaluable injections of enthusiasm, advice, and friendship. I extend my most sincere thank you to Dr. Burkhard Pohl, The Big Hom Basin Foundation, and the Wyoming Dinosaur Center. Without whose input and financial support this thesis would not have been possible. In conjunction I would like to thank the staff of the Wyoming Dinosaur Center for the great deal of help that I received during my stay in Thermopolis. Finally I wish to thank my friends and family. To my friends who have pursued this process before me, and with me; thank you very much. -

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

A Dated Phylogeny of Marsupials Using a Molecular Supermatrix and Multiple Fossil Constraints
Journal of Mammalogy, 89(1):175–189, 2008 A DATED PHYLOGENY OF MARSUPIALS USING A MOLECULAR SUPERMATRIX AND MULTIPLE FOSSIL CONSTRAINTS ROBIN M. D. BECK* School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, New South Wales 2052, Australia Downloaded from https://academic.oup.com/jmammal/article/89/1/175/1020874 by guest on 25 September 2021 Phylogenetic relationships within marsupials were investigated based on a 20.1-kilobase molecular supermatrix comprising 7 nuclear and 15 mitochondrial genes analyzed using both maximum likelihood and Bayesian approaches and 3 different partitioning strategies. The study revealed that base composition bias in the 3rd codon positions of mitochondrial genes misled even the partitioned maximum-likelihood analyses, whereas Bayesian analyses were less affected. After correcting for base composition bias, monophyly of the currently recognized marsupial orders, of Australidelphia, and of a clade comprising Dasyuromorphia, Notoryctes,and Peramelemorphia, were supported strongly by both Bayesian posterior probabilities and maximum-likelihood bootstrap values. Monophyly of the Australasian marsupials, of Notoryctes þ Dasyuromorphia, and of Caenolestes þ Australidelphia were less well supported. Within Diprotodontia, Burramyidae þ Phalangeridae received relatively strong support. Divergence dates calculated using a Bayesian relaxed molecular clock and multiple age constraints suggested at least 3 independent dispersals of marsupials from North to South America during the Late Cretaceous or early Paleocene. Within the Australasian clade, the macropodine radiation, the divergence of phascogaline and dasyurine dasyurids, and the divergence of perameline and peroryctine peramelemorphians all coincided with periods of significant environmental change during the Miocene. An analysis of ‘‘unrepresented basal branch lengths’’ suggests that the fossil record is particularly poor for didelphids and most groups within the Australasian radiation. -
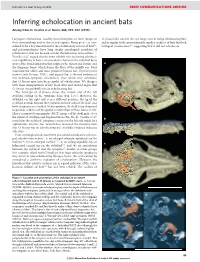
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -
Reptile Family Tree
Reptile Family Tree - Peters 2015 Distribution of Scales, Scutes, Hair and Feathers Fish scales 100 Ichthyostega Eldeceeon 1990.7.1 Pederpes 91 Eldeceeon holotype Gephyrostegus watsoni Eryops 67 Solenodonsaurus 87 Proterogyrinus 85 100 Chroniosaurus Eoherpeton 94 72 Chroniosaurus PIN3585/124 98 Seymouria Chroniosuchus Kotlassia 58 94 Westlothiana Casineria Utegenia 84 Brouffia 95 78 Amphibamus 71 93 77 Coelostegus Cacops Paleothyris Adelospondylus 91 78 82 99 Hylonomus 100 Brachydectes Protorothyris MCZ1532 Eocaecilia 95 91 Protorothyris CM 8617 77 95 Doleserpeton 98 Gerobatrachus Protorothyris MCZ 2149 Rana 86 52 Microbrachis 92 Elliotsmithia Pantylus 93 Apsisaurus 83 92 Anthracodromeus 84 85 Aerosaurus 95 85 Utaherpeton 82 Varanodon 95 Tuditanus 91 98 61 90 Eoserpeton Varanops Diplocaulus Varanosaurus FMNH PR 1760 88 100 Sauropleura Varanosaurus BSPHM 1901 XV20 78 Ptyonius 98 89 Archaeothyris Scincosaurus 77 84 Ophiacodon 95 Micraroter 79 98 Batropetes Rhynchonkos Cutleria 59 Nikkasaurus 95 54 Biarmosuchus Silvanerpeton 72 Titanophoneus Gephyrostegeus bohemicus 96 Procynosuchus 68 100 Megazostrodon Mammal 88 Homo sapiens 100 66 Stenocybus hair 91 94 IVPP V18117 69 Galechirus 69 97 62 Suminia Niaftasuchus 65 Microurania 98 Urumqia 91 Bruktererpeton 65 IVPP V 18120 85 Venjukovia 98 100 Thuringothyris MNG 7729 Thuringothyris MNG 10183 100 Eodicynodon Dicynodon 91 Cephalerpeton 54 Reiszorhinus Haptodus 62 Concordia KUVP 8702a 95 59 Ianthasaurus 87 87 Concordia KUVP 96/95 85 Edaphosaurus Romeria primus 87 Glaucosaurus Romeria texana Secodontosaurus -

HOVASAURUS BOULEI, an AQUATIC EOSUCHIAN from the UPPER PERMIAN of MADAGASCAR by P.J
99 Palaeont. afr., 24 (1981) HOVASAURUS BOULEI, AN AQUATIC EOSUCHIAN FROM THE UPPER PERMIAN OF MADAGASCAR by P.J. Currie Provincial Museum ofAlberta, Edmonton, Alberta, T5N OM6, Canada ABSTRACT HovasauTUs is the most specialized of four known genera of tangasaurid eosuchians, and is the most common vertebrate recovered from the Lower Sakamena Formation (Upper Per mian, Dzulfia n Standard Stage) of Madagascar. The tail is more than double the snout-vent length, and would have been used as a powerful swimming appendage. Ribs are pachyostotic in large animals. The pectoral girdle is low, but massively developed ventrally. The front limb would have been used for swimming and for direction control when swimming. Copious amounts of pebbles were swallowed for ballast. The hind limbs would have been efficient for terrestrial locomotion at maturity. The presence of long growth series for Ho vasaurus and the more terrestrial tan~saurid ThadeosauTUs presents a unique opportunity to study differences in growth strategies in two closely related Permian genera. At birth, the limbs were relatively much shorter in Ho vasaurus, but because of differences in growth rates, the limbs of Thadeosau rus are relatively shorter at maturity. It is suggested that immature specimens of Ho vasauTUs spent most of their time in the water, whereas adults spent more time on land for mating, lay ing eggs and/or range dispersal. Specilizations in the vertebrae and carpus indicate close re lationship between Youngina and the tangasaurids, but eliminate tangasaurids from consider ation as ancestors of other aquatic eosuchians, archosaurs or sauropterygians. CONTENTS Page ABREVIATIONS . ..... ... ......... .......... ... ......... ..... ... ..... .. .... 101 INTRODUCTION . -

Constraints on the Timescale of Animal Evolutionary History
Palaeontologia Electronica palaeo-electronica.org Constraints on the timescale of animal evolutionary history Michael J. Benton, Philip C.J. Donoghue, Robert J. Asher, Matt Friedman, Thomas J. Near, and Jakob Vinther ABSTRACT Dating the tree of life is a core endeavor in evolutionary biology. Rates of evolution are fundamental to nearly every evolutionary model and process. Rates need dates. There is much debate on the most appropriate and reasonable ways in which to date the tree of life, and recent work has highlighted some confusions and complexities that can be avoided. Whether phylogenetic trees are dated after they have been estab- lished, or as part of the process of tree finding, practitioners need to know which cali- brations to use. We emphasize the importance of identifying crown (not stem) fossils, levels of confidence in their attribution to the crown, current chronostratigraphic preci- sion, the primacy of the host geological formation and asymmetric confidence intervals. Here we present calibrations for 88 key nodes across the phylogeny of animals, rang- ing from the root of Metazoa to the last common ancestor of Homo sapiens. Close attention to detail is constantly required: for example, the classic bird-mammal date (base of crown Amniota) has often been given as 310-315 Ma; the 2014 international time scale indicates a minimum age of 318 Ma. Michael J. Benton. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Philip C.J. Donoghue. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Robert J. -

Mammal Faunal Change in the Zone of the Paleogene Hyperthermals ETM2 and H2
Clim. Past, 11, 1223–1237, 2015 www.clim-past.net/11/1223/2015/ doi:10.5194/cp-11-1223-2015 © Author(s) 2015. CC Attribution 3.0 License. Mammal faunal change in the zone of the Paleogene hyperthermals ETM2 and H2 A. E. Chew Department of Anatomy, Western University of Health Sciences, 309 E Second St., Pomona, CA 91767, USA Correspondence to: A. E. Chew ([email protected]) Received: 13 March 2015 – Published in Clim. Past Discuss.: 16 April 2015 Revised: 4 August 2015 – Accepted: 19 August 2015 – Published: 24 September 2015 Abstract. “Hyperthermals” are past intervals of geologically vulnerability in response to changes already underway in the rapid global warming that provide the opportunity to study lead-up to the EECO. Faunal response at faunal events B-1 the effects of climate change on existing faunas over thou- and B-2 is also distinctive in that it shows high proportions sands of years. A series of hyperthermals is known from of beta richness, suggestive of increased geographic disper- the early Eocene ( ∼ 56–54 million years ago), including sal related to transient increases in habitat (floral) complexity the Paleocene–Eocene Thermal Maximum (PETM) and two and/or precipitation or seasonality of precipitation. subsequent hyperthermals (Eocene Thermal Maximum 2 – ETM2 – and H2). The later hyperthermals occurred during warming that resulted in the Early Eocene Climatic Opti- mum (EECO), the hottest sustained period of the Cenozoic. 1 Introduction The PETM has been comprehensively studied in marine and terrestrial settings, but the terrestrial biotic effects of ETM2 The late Paleocene and early Eocene (ca. -

(Diapsida: Saurosphargidae), with Implications for the Morphological Diversity and Phylogeny of the Group
Geol. Mag.: page 1 of 21. c Cambridge University Press 2013 1 doi:10.1017/S001675681300023X A new species of Largocephalosaurus (Diapsida: Saurosphargidae), with implications for the morphological diversity and phylogeny of the group ∗ CHUN LI †, DA-YONG JIANG‡, LONG CHENG§, XIAO-CHUN WU†¶ & OLIVIER RIEPPEL ∗ Laboratory of Evolutionary Systematics of Vertebrates, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, PO Box 643, Beijing 100044, China ‡Department of Geology and Geological Museum, Peking University, Beijing 100871, PR China §Wuhan Institute of Geology and Mineral Resources, Wuhan, 430223, PR China ¶Canadian Museum of Nature, PO Box 3443, STN ‘D’, Ottawa, ON K1P 6P4, Canada Department of Geology, The Field Museum, 1400 S. Lake Shore Drive, Chicago, IL 60605-2496, USA (Received 31 July 2012; accepted 25 February 2013) Abstract – Largocephalosaurus polycarpon Cheng et al. 2012a was erected after the study of the skull and some parts of a skeleton and considered to be an eosauropterygian. Here we describe a new species of the genus, Largocephalosaurus qianensis, based on three specimens. The new species provides many anatomical details which were described only briefly or not at all in the type species, and clearly indicates that Largocephalosaurus is a saurosphargid. It differs from the type species mainly in having three premaxillary teeth, a very short retroarticular process, a large pineal foramen, two sacral vertebrae, and elongated small granular osteoderms mixed with some large ones along the lateral most side of the body. With additional information from the new species, we revise the diagnosis and the phylogenetic relationships of Largocephalosaurus and clarify a set of diagnostic features for the Saurosphargidae Li et al. -

Implications for Predatory Dinosaur Macroecology and Ontogeny in Later Late Cretaceous Asiamerica
Canadian Journal of Earth Sciences Theropod Guild Structure and the Tyrannosaurid Niche Assimilation Hypothesis: Implications for Predatory Dinosaur Macroecology and Ontogeny in later Late Cretaceous Asiamerica Journal: Canadian Journal of Earth Sciences Manuscript ID cjes-2020-0174.R1 Manuscript Type: Article Date Submitted by the 04-Jan-2021 Author: Complete List of Authors: Holtz, Thomas; University of Maryland at College Park, Department of Geology; NationalDraft Museum of Natural History, Department of Geology Keyword: Dinosaur, Ontogeny, Theropod, Paleocology, Mesozoic, Tyrannosauridae Is the invited manuscript for consideration in a Special Tribute to Dale Russell Issue? : © The Author(s) or their Institution(s) Page 1 of 91 Canadian Journal of Earth Sciences 1 Theropod Guild Structure and the Tyrannosaurid Niche Assimilation Hypothesis: 2 Implications for Predatory Dinosaur Macroecology and Ontogeny in later Late Cretaceous 3 Asiamerica 4 5 6 Thomas R. Holtz, Jr. 7 8 Department of Geology, University of Maryland, College Park, MD 20742 USA 9 Department of Paleobiology, National Museum of Natural History, Washington, DC 20013 USA 10 Email address: [email protected] 11 ORCID: 0000-0002-2906-4900 Draft 12 13 Thomas R. Holtz, Jr. 14 Department of Geology 15 8000 Regents Drive 16 University of Maryland 17 College Park, MD 20742 18 USA 19 Phone: 1-301-405-4084 20 Fax: 1-301-314-9661 21 Email address: [email protected] 22 23 1 © The Author(s) or their Institution(s) Canadian Journal of Earth Sciences Page 2 of 91 24 ABSTRACT 25 Well-sampled dinosaur communities from the Jurassic through the early Late Cretaceous show 26 greater taxonomic diversity among larger (>50kg) theropod taxa than communities of the 27 Campano-Maastrichtian, particularly to those of eastern/central Asia and Laramidia. -

Conceptual Issues in Phylogeny, Taxonomy, and Nomenclature
Contributions to Zoology, 66 (1) 3-41 (1996) SPB Academic Publishing bv, Amsterdam Conceptual issues in phylogeny, taxonomy, and nomenclature Alexandr P. Rasnitsyn Paleontological Institute, Russian Academy ofSciences, Profsoyuznaya Street 123, J17647 Moscow, Russia Keywords: Phylogeny, taxonomy, phenetics, cladistics, phylistics, principles of nomenclature, type concept, paleoentomology, Xyelidae (Vespida) Abstract On compare les trois approches taxonomiques principales développées jusqu’à présent, à savoir la phénétique, la cladis- tique et la phylistique (= systématique évolutionnaire). Ce Phylogenetic hypotheses are designed and tested (usually in dernier terme s’applique à une approche qui essaie de manière implicit form) on the basis ofa set ofpresumptions, that is, of à les traits fondamentaux de la taxonomic statements explicite représenter describing a certain order of things in nature. These traditionnelle en de leur et particulier son usage preuves ayant statements are to be accepted as such, no matter whatever source en même temps dans la similitude et dans les relations de evidence for them exists, but only in the absence ofreasonably parenté des taxons en question. L’approche phylistique pré- sound evidence pleading against them. A set ofthe most current sente certains avantages dans la recherche de réponses aux phylogenetic presumptions is discussed, and a factual example problèmes fondamentaux de la taxonomie. ofa practical realization of the approach is presented. L’auteur considère la nomenclature A is made of the three -

Appendix 1 – Environmental Predictor Data
APPENDIX 1 – ENVIRONMENTAL PREDICTOR DATA CONTENTS Overview ..................................................................................................................................................................................... 2 Climate ......................................................................................................................................................................................... 2 Hydrology ................................................................................................................................................................................... 3 Land Use and Land Cover ..................................................................................................................................................... 3 Soils and Substrate .................................................................................................................................................................. 5 Topography .............................................................................................................................................................................. 10 References ................................................................................................................................................................................ 12 1 OVERVIEW A set of 94 potential predictor layers compiled to use in distribution modeling for the target taxa. Many of these layers derive from previous modeling work by WYNDD1, 2, but a