Smoking Cessation: a Report of the Surgeon General
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CLINICAL TRIALS Safety and Immunogenicity of a Nicotine Conjugate Vaccine in Current Smokers
CLINICAL TRIALS Safety and immunogenicity of a nicotine conjugate vaccine in current smokers Immunotherapy is a novel potential treatment for nicotine addiction. The aim of this study was to assess the safety and immunogenicity of a nicotine conjugate vaccine, NicVAX, and its effects on smoking behavior. were recruited for a noncessation treatment study and assigned to 1 of 3 doses of the (68 ؍ Smokers (N nicotine vaccine (50, 100, or 200 g) or placebo. They were injected on days 0, 28, 56, and 182 and monitored for a period of 38 weeks. Results showed that the nicotine vaccine was safe and well tolerated. Vaccine immunogenicity was dose-related (P < .001), with the highest dose eliciting antibody concentrations within the anticipated range of efficacy. There was no evidence of compensatory smoking or precipitation of nicotine withdrawal with the nicotine vaccine. The 30-day abstinence rate was significantly different across with the highest rate of abstinence occurring with 200 g. The nicotine vaccine appears ,(02. ؍ the 4 doses (P to be a promising medication for tobacco dependence. (Clin Pharmacol Ther 2005;78:456-67.) Dorothy K. Hatsukami, PhD, Stephen Rennard, MD, Douglas Jorenby, PhD, Michael Fiore, MD, MPH, Joseph Koopmeiners, Arjen de Vos, MD, PhD, Gary Horwith, MD, and Paul R. Pentel, MD Minneapolis, Minn, Omaha, Neb, Madison, Wis, and Rockville, Md Surveys show that, although about 41% of smokers apy, is about 25% on average.2 Moreover, these per- make a quit attempt each year, less than 5% of smokers centages most likely exaggerate the efficacy of are successful at remaining abstinent for 3 months to a intervention because these trials are typically composed year.1 Smokers seeking available behavioral and phar- of subjects who are highly motivated to quit and who macologic therapies can enhance successful quit rates are free of complicating diagnoses such as depression 2 by 2- to 3-fold over control conditions. -

Neurocops: the Politics of Prohibition and the Future of Enforcing Social Policy from Inside the Body
NEUROCOPS: THE POLITICS OF PROHIBITION AND THE FUTURE OF ENFORCING SOCIAL POLICY FROM INSIDE THE BODY RICHARD GLEN BOIRE1 I. INTRODUCTION .................................................................... 216 II. FROM DEMAND REDUCTION TO DESIRE REDUCTION.......................................................................... 216 III. PHARMACOTHERAPY DRUGS ............................................... 218 A. Target: Opiates............................................................ 218 B. Target: Cocaine........................................................... 221 C. Target: Marijuana....................................................... 222 D. Targeting Legal Drugs ................................................ 223 1. Target: Nicotine.................................................... 223 2. Target: Alcohol..................................................... 225 E. Pharmacotherapy Drugs: Good, Bad, Both, or Beyond?......................................................... 225 F. From Drug War to Drug Epidemic ............................. 230 IV. NEUROCOPS: LEGAL ISSUES RAISED BY COMPULSORY PHARMACOTHERAPY..................................... 234 A. Privacy and Liberty Interests Implicated by Involuntary Pharamacotherapy.............................. 234 B. Informed Consent......................................................... 236 C. At Risk Targets for Coercive Pharmacotherapy ........................................................ 238 1. Pharmacotherapy and Public Education............................................................. -
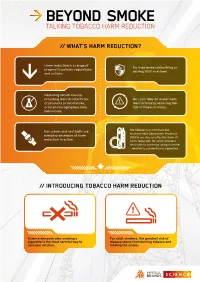
Talking Tobacco Harm Reduction
TALKING TOBACCO HARM REDUCTION // WHAT’S HARM REDUCTION? Harm reduction is a range of Do they make sunbathing or pragmatic policies, regulations driving 100% risk-free? and actions. Reducing health risks by providing less harmful forms No – but they do make them of products or substances, less harmful by reducing the or by encouraging less risky risk of illness or injury. behaviours. Sun cream and seat belts are We believe non-combustible nicotine Next Generation Products everyday examples of harm (NGPs) are also an effective form of reduction in action. harm reduction for adult smokers who wish to continue using nicotine – relative to conventional cigarettes. // INTRODUCING TOBACCO HARM REDUCTION Science demonstrates smoking a For adult smokers, the greatest risk of cigarette is the most harmful way to disease stems from burning tobacco and consume nicotine. inhaling the smoke. // WHAT IS THR? Tobacco smoke contains over 7000 The undisputed best action adult chemicals – nicotine is one of them. smokers can take to improve their Around 100 are classified by public health is to stop all tobacco and health experts as causes or potential nicotine use entirely, but many are not causes of smoking-related disease. interested or willing to take this step. Numerous public health bodies1 While the science suggests nicotine is believe transitioning to nicotine addictive and not risk-free, it’s neither products that are substantially less carcinogenic nor the primary cause of harmful than inhaled tobacco smoke smoking-related diseases. is their next best option – we agree. Contains 7000+ Contains chemicals, 100 significantly of them harmful fewer and lower or potentially levels of harmful harmful. -

Are Smoking, Environmental Pollution, and Weather Conditions Risk Factors for COVID-19? José Miguel Chatkin1a, Irma Godoy2a
J Bras Pneumol. 2020;46(5):e20200183 https://dx.doi.org/10.36416/1806-3756/e20200183 REVIEW ARTICLE Are smoking, environmental pollution, and weather conditions risk factors for COVID-19? José Miguel Chatkin1a, Irma Godoy2a 1. Departamento de Medicina Interna ABSTRACT e Pneumologia, Escola de Medicina, Pontifícia Universidade Católica do Rio Coronavirus disease 2019 (COVID-19), caused by the highly contagious severe acute Grande do Sul, Porto Alegre (RS) Brasil. respiratory syndrome coronavirus 2 (SARS-CoV-2), is probably systemic, has a major 2. Disciplina de Pneumologia, respiratory component, and is transmitted by person-to-person contact, via airborne Departamento de Clínica Médica, droplets or aerosols. In the respiratory tract, the virus begins to replicate within cells, Faculdade de Medicina de Botucatu, after which the host starts shedding the virus. The individuals recognized as being at risk Universidade Estadual Paulista, Botucatu (SP) Brasil. for an unfavorable COVID-19 outcome are those > 60 years of age, those with chronic diseases such as diabetes mellitus, those with hypertension, and those with chronic lung Submitted: 20 April 2020. diseases, as well as those using chemotherapy, corticosteroids, or biological agents. Accepted: 27 May 2020. Some studies have suggested that infection with SARS-CoV-2 is associated with other Study carried out at the Escola de risk factors, such as smoking, external environmental pollution, and certain climatic Medicina, Pontifícia Universidade Católica conditions. The purpose of this narrative review was to perform a critical assessment of do Rio Grande do Sul, Porto Alegre (RS) the relationship between COVID-19 and these potential risk factors. and at the Faculdade de Medicina de Botucatu, Universidade Estadual Paulista, Keywords: Coronavirus infections; COVID-19; Air pollution; Smoking; Tobacco use Botucatu (SP) Brasil. -

Modulation of Immune Responses Using Adjuvants to Facilitate Therapeutic Vaccination
Received: 6 March 2020 | Revised: 30 April 2020 | Accepted: 20 May 2020 DOI: 10.1111/imr.12889 INVITED REVIEW Modulation of immune responses using adjuvants to facilitate therapeutic vaccination Virgil Schijns1 | Alberto Fernández-Tejada2,3 | Žarko Barjaktarović4 | Ilias Bouzalas5 | Jens Brimnes6 | Sergey Chernysh7,† | Sveinbjorn Gizurarson8 | Ihsan Gursel9 | Žiga Jakopin10 | Maria Lawrenz11 | Cristina Nativi12 | Stephane Paul13 | Gabriel Kristian Pedersen14 | Camillo Rosano15 | Ane Ruiz-de-Angulo2 | Bram Slütter16 | Aneesh Thakur17 | Dennis Christensen14 | Ed C. Lavelle18 1Wageningen University, Cell Biology & Immunology and, ERC-The Netherlands, Schaijk, Landerd campus, The Netherlands 2Chemical Immunology Lab, Center for Cooperative Research in Biosciences, CIC bioGUNE, Biscay, Spain 3Ikerbasque, Basque Foundation for Science, Bilbao, Spain 4Agency for Medicines and Medical Devices of Montenegro, Podgorica, Montenegro 5Hellenic Agricultural Organization-DEMETER, Veterinary Research Institute, Thessaloniki, Greece 6Alk Abello, Copenhagen, Denmark 7Laboratory of Insect Biopharmacology and Immunology, Department of Entomology, Saint-Petersburg State University, Saint-Petersburg, Russia 8Faculty of Pharmaceutical Sciences, University of Iceland, Reykjavik, Iceland 9Bilkent University, Ankara, Turkey 10Faculty of Pharmacy, University of Ljubljana, Ljubljana, Slovenia 11Vaccine Formulation Institute (CH), Geneva, Switzerland 12Department of Chemistry, University of Florence, Florence, Italy 13St Etienne University, St Etienne, France 14Statens -

Comments to the FDA on Swedish Match
November 25, 2014 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Docket No. FDA-2014-N-1051, Modified Risk Tobacco Product Applications: Applications for 10 Products Submitted by Swedish Match North America, Inc. The undersigned organizations submit these comments in response to the announcement for public comment of a modified risk tobacco product application by Swedish Match North America, Inc. (“Swedish Match” or “the company”) for 10 tobacco products.1 The Swedish Match application presents evidence to FDA that support the proposition that Swedish smokers who switch entirely to snus derive individual health benefits. Swedish Match also provides evidence indicating that the high prevalence of snus usage among men in Sweden has contributed to a lower frequency of tobacco-related disease and mortality than is found in comparable populations with higher cigarette smoking rates. This information suggests that if Swedish Match presents convincing evidence that being permitted to make a modified risk claim would lead smokers that would not otherwise quit smoking to switch completely to one of these Swedish snus products, without leading to significant use among youth, such an application would deserve serious consideration. However, as discussed at length in Part I below, the modified risk application submitted by Swedish Match in its current form must be denied by FDA because it is legally defective under federal law. Although Swedish Match seeks a modified risk order under Section 911 of the Food, Drug & Cosmetic Act (FDCA), as amended by the Family Smoking Prevention and Tobacco Control Act (“Tobacco Control Act” or “TCA”), Swedish Match does not propose to make a claim concerning the relative risk for the listed snus products. -

An Endgame for Tobacco? by the Various Proposals
Editorial Tob Control: first published as 10.1136/tobaccocontrol-2013-050989 on 15 April 2013. Downloaded from then to evaluate what might be achieved An endgame for tobacco? by the various proposals. The latter requires contemplation of each strategy’s Kenneth E Warner potential outcomes, good and undesirable, and the political, legal, ethical, economic, regulatory and social barriers to realising ABSTRACT great successes of many national anti- the strategy’s implementation. Finally, the Since its origins in the 1960s, tobacco control smoking campaigns notwithstanding, the very idea of what constitutes the end of has achieved remarkable success against the pandemic persists. In many developed the endgame needs to be addressed. scourge of tobacco-produced disease and countries with the most robust tobacco Strikingly, to date, there has never been a death. Yet tobacco use, especially cigarette control efforts, the decline in smoking serious discussion within the tobacco smoking, remains the world's leading cause of prevalence has slowed to a trickle. control community about what would preventable premature death and is likely to Evidence-based policies seem incapable of constitute a final victory in tobacco do so for decades to come. Evidence-based substantially hastening the demise of control. Would it be a reduction in 5 policies seem incapable of substantially smoking in these countries. In LMICs, smoking-produced deaths to, say, 10% of hastening the demise of smoking. Slowness in smoking is on the rise. The net effect is current levels? -

Trends in Cigarette Smoking Cessation in the United States
Tobacco Control 1993; 2 (suppl): S3-S16 S3 SESSION I TRENDS IN CESSATION Tob Control: first published as 10.1136/tc.2.suppl1.S4 on 1 January 1993. Downloaded from Introduction Saul Shiftman I'm happy to welcome you here on behalf of of the National Heart, Lung and Blood the Planning Committee. It's a pleasure to see Institute's Smoking Education Program, this actually happening after almost a year of whose job it is to translate findings from planning, and I'm looking forward to the next intervention research into community action day and a half. and education programmes; he was formerly We have 2454 days left until the year 2000, the director of smoking intervention with the at which point the national goals are to have a American Heart Association. smoking prevalence of 15%. We're now at Dr Ellen Gritz is a long-time colleague of roughly 25 %. Put another way, we are 81 % of mine, although I hesitate to remind her in the way to the year 2000 from 1964, the year of public that she and I first started working the first Surgeon General's report, and we together a little over 20 years ago in Los have cut smoking prevalence roughly by half. Angeles. She is certainly one of the leading So we're doing pretty well but still have a bit experts in smoking and smoking cessation, and further to go. has particular interests in smoking among In this morning's panel, we'll be discussing women and in special populations. where we're going and how we're going to get Our last panellist, Dr Patrick O'Malley is there in terms of smoking cessation. -

Primary Care & Tobacco Cessation Handbook: a Reference for Providers
Primary Care & Tobacco Cessation Handbook A Resource for Providers Primary Care & Tobacco Cessation Handbook A Resource for Providers Acknowledgements The provider manual Primary Care & Tobacco Cessation and the accompanying My Tobacco Cessation Workbook were developed by Julianne Himstreet, Pharm.D., BCPS. The author’s primary goal was to develop materials promoting tobacco cessation interventions, based on published principles of evidence- and consensus- based clinical practice, for use by primary care providers treating patients who use tobacco. With permission from the HIV and Smoking Cessation (HASC) Working Group, several materials used in the Primary Care & Tobacco Cessation provider manual were modified from HIV Provider Smoking Cessation Handbook. The U.S. Public Health Service Clinical Practice Guideline (Fiore, 2000) and the treatment model described by Richard Brown (2003) provided the foundation for their work and therefore indirectly ours as well.1 Many thanks to Kim Hamlett- Berry, Director of Tobacco & Health Policy in VHA Office of Mental Health Services for supporting this project and Leah Stockett for editing the manual and the workbook. In addition, much appreciation needs to be given to Dana Christofferson, Kim Hamlett- Berry, Pam Belperio, and Tim Chen for their editing and content contributions.1 1 Brown, R. A. (2003). Intensive behavioral treatment. In D. B. Abrams, R. Niaura, R. Brown, K. M. Emmons, M. G. Goldstein, & P. M. Monti, The tobacco dependence treatment handbook: A guide to best practices (pp. 118 -177). New York, NY: Guilford Press. Fiore, M. C., Bailey, W. C., Cohen, S. J., Dorfman, S. F., Goldstein, M. G., Gritz, E. R., Heyman, R. -

Cigarettes and Tobacco Products Removed from the California Tobacco Directory by Brand
Cigarettes and Tobacco Products Removed From The California Tobacco Directory by Brand Brand Manufacturer Date Comments Removed #117 - RYO National Tobacco Company 10/21/2011 5/6/05 Man. Change from RBJ to National Tobacco Company 10/20's (ten-twenty's) M/s Dhanraj International 2/6/2012 2/2/05 Man. Name change from Dhanraj Imports, Inc. 10/20's (ten-twenty's) - RYO M/s Dhanraj International 2/6/2012 1st Choice R.J. Reynolds Tobacco Company 5/3/2010 Removed 5/2/08; Reinstated 7/11/08 32 Degrees General Tobacco 2/28/2010 4 Aces - RYO Top Tobacco, LP 11/12/2010 A Touch of Clove Sherman 1400 Broadway N.Y.C. Inc. 9/25/2009 AB Rimboche' - RYO Daughters & Ryan, Inc. 6/18/2010 Ace King Maker Marketing 5/21/2020 All American Value Philip Morris, USA 5/5/2006 All Star Liberty Brands, LLC 5/5/2006 Alpine Philip Morris, USA 8/14/2013 Removed 5/4/07; Reinstated 5/8/09 Always Save Liberty Brands, LLC 5/4/2007 American R.J. Reynolds Tobacco Company 5/6/2005 American Bison Wind River Tobacco Company, LLC 9/22/2015 American Blend Mac Baren Tobacco Company 5/4/2007 American Harvest Sandia Tobacco Manufacturers, Inc. 8/31/2016 American Harvest - RYO Truth & Liberty Manufacturing 8/2/2016 American Liberty Les Tabacs Spokan 5/12/2006 Amphora - RYO Top Tobacco, LP 11/18/2011 Andron's Passion VCT 5/4/2007 Andron's Passion VCT 5/4/2007 Arango Sportsman - RYO Daughters & Ryan, Inc. 6/18/2010 Arbo - RYO VCT 5/4/2007 Ashford Von Eicken Group 5/8/2009 Ashford - RYO Von Eicken Group 12/23/2011 Athey (Old Timer's) Daughters & Ryan, Inc. -
Tobacco in Canada Addressing Knowledge Gaps Important to Tobacco Regulation Environmental Scan – Autumn 2020
Tobacco in Canada Addressing Knowledge Gaps Important to Tobacco Regulation Environmental Scan – Autumn 2020 Vuse flagship store opens in Toronto, October 2019. December 31, 2020 Physicians for a Smoke-Free Canada 134 Caroline Avenue Ottawa Ontario K1Y 0S9 www.smoke-free.ca psc @ smoke-free.ca TABLE OF CONTENTS Table of Contents .................................................................................................................................................. 2 I. Federal Government Activities .......................................................................................................................... 3 a) Policy and Regulation.................................................................................................................................... 3 B) Financial Policy ............................................................................................................................................. 5 II. Monitoring and Surveillance ............................................................................................................................. 7 III. Provincial Government activities ..................................................................................................................... 8 New Brunswick ................................................................................................................................................. 8 Newfoundland ................................................................................................................................................. -

Summary of Product Characteristics 1. Name Of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Nicorette Mint 2 mg Compressed Lozenge 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each lozenge contains 2 mg nicotine (as nicotine resinate) For a full list of excipients, see section 6.1 3. PHARMACEUTICAL FORM Compressed Lozenge An oval, white to off-white tablet imprinted with an “n” on one side and “2” on the other side 4. CLINICAL PARTICULARS 4.1 Therapeutic Indications Nicorette Mint 2 mg Lozenges are to be used for the treatment of tobacco dependence by relief of nicotine withdrawal symptoms and cravings in smokers 18 years and above. Permanent cessation of tobacco use is the eventual objective. Nicorette Mint 2 mg Lozenges should preferably be used in conjunction with a behavioural support programme. Use of Nicorette Mint 2 mg Lozenge in smokers 12 – 17 years inclusive is at the discretion of a doctor: See section 4.2. 4.2 Posology and Method of Administration Posology Selecting the strength of lozenge to be used will depend on the smoking habits of the individual. Adults Nicorette Mint 2 mg Lozenges are suitable for smokers with low nicotine dependency e.g. those smoking their first cigarette of the day more than 30 minutes after waking up or those who smoke fewer than 20 cigarettes per day. Lozenges should not be used for more than 9 months. If users still feel the need for treatment, a physician should be consulted. Behavioural therapy advice and support will normally improve the success rate. 1/9 Paediatric population Children and adolescents Nicorette Mint 2 mg Lozenges should only be used by adolescents (12-17 years inclusive) with advice from a doctor.