Lecture 2: Atmospheric Thermodynamics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heat Advection Processes Leading to El Niño Events As
1 2 Title: 3 Heat advection processes leading to El Niño events as depicted by an ensemble of ocean 4 assimilation products 5 6 Authors: 7 Joan Ballester (1,2), Simona Bordoni (1), Desislava Petrova (2), Xavier Rodó (2,3) 8 9 Affiliations: 10 (1) California Institute of Technology (Caltech), Pasadena, California, United States 11 (2) Institut Català de Ciències del Clima (IC3), Barcelona, Catalonia, Spain 12 (3) Institució Catalana de Recerca i Estudis Avançats (ICREA), Barcelona, Catalonia, Spain 13 14 Corresponding author: 15 Joan Ballester 16 California Institute of Technology (Caltech) 17 1200 E California Blvd, Pasadena, CA 91125, US 18 Mail Code: 131-24 19 Tel.: +1-626-395-8703 20 Email: [email protected] 21 22 Manuscript 23 Submitted to Journal of Climate 24 1 25 26 Abstract 27 28 The oscillatory nature of El Niño-Southern Oscillation results from an intricate 29 superposition of near-equilibrium balances and out-of-phase disequilibrium processes between the 30 ocean and the atmosphere. Several authors have shown that the heat content stored in the equatorial 31 subsurface is key to provide memory to the system. Here we use an ensemble of ocean assimilation 32 products to describe how heat advection is maintained in each dataset during the different stages of 33 the oscillation. 34 Our analyses show that vertical advection due to surface horizontal convergence and 35 downwelling motion is the only process contributing significantly to the initial subsurface warming 36 in the western equatorial Pacific. This initial warming is found to be advected to the central Pacific 37 by the equatorial undercurrent, which, together with the equatorward advection associated with 38 anomalies in both the meridional temperature gradient and circulation at the level of the 39 thermocline, explains the heat buildup in the central Pacific during the recharge phase. -

3-1 Adiabatic Compression
Solution Physics 213 Problem 1 Week 3 Adiabatic Compression a) Last week, we considered the problem of isothermal compression: 1.5 moles of an ideal diatomic gas at temperature 35oC were compressed isothermally from a volume of 0.015 m3 to a volume of 0.0015 m3. The pV-diagram for the isothermal process is shown below. Now we consider an adiabatic process, with the same starting conditions and the same final volume. Is the final temperature higher, lower, or the same as the in isothermal case? Sketch the adiabatic processes on the p-V diagram below and compute the final temperature. (Ignore vibrations of the molecules.) α α For an adiabatic process ViTi = VfTf , where α = 5/2 for the diatomic gas. In this case Ti = 273 o 1/α 2/5 K + 35 C = 308 K. We find Tf = Ti (Vi/Vf) = (308 K) (10) = 774 K. b) According to your diagram, is the final pressure greater, lesser, or the same as in the isothermal case? Explain why (i.e., what is the energy flow in each case?). Calculate the final pressure. We argued above that the final temperature is greater for the adiabatic process. Recall that p = nRT/ V for an ideal gas. We are given that the final volume is the same for the two processes. Since the final temperature is greater for the adiabatic process, the final pressure is also greater. We can do the problem numerically if we assume an idea gas with constant α. γ In an adiabatic processes, pV = constant, where γ = (α + 1) / α. -

Thermodynamics of Solar Energy Conversion in to Work
Sri Lanka Journal of Physics, Vol. 9 (2008) 47-60 Institute of Physics - Sri Lanka Research Article Thermodynamic investigation of solar energy conversion into work W.T.T. Dayanga and K.A.I.L.W. Gamalath Department of Physics, University of Colombo, Colombo 03, Sri Lanka Abstract Using a simple thermodynamic upper bound efficiency model for the conversion of solar energy into work, the best material for a converter was obtained. Modifying the existing detailed terrestrial application model of direct solar radiation to include an atmospheric transmission coefficient with cloud factors and a maximum concentration ratio, the best shape for a solar concentrator was derived. Using a Carnot engine in detailed space application model, the best shape for the mirror of a concentrator was obtained. A new conversion model was introduced for a solar chimney power plant to obtain the efficiency of the power plant and power output. 1. INTRODUCTION A system that collects and converts solar energy in to mechanical or electrical power is important from various aspects. There are two major types of solar power systems at present, one using photovoltaic cells for direct conversion of solar radiation energy in to electrical energy in combination with electrochemical storage and the other based on thermodynamic cycles. The efficiency of a solar thermal power plant is significantly higher [1] compared to the maximum efficiency of twenty percent of a solar cell. Although the initial cost of a solar thermal power plant is very high, the running cost is lower compared to the other power plants. Therefore most countries tend to build solar thermal power plants. -

Soaring Weather
Chapter 16 SOARING WEATHER While horse racing may be the "Sport of Kings," of the craft depends on the weather and the skill soaring may be considered the "King of Sports." of the pilot. Forward thrust comes from gliding Soaring bears the relationship to flying that sailing downward relative to the air the same as thrust bears to power boating. Soaring has made notable is developed in a power-off glide by a conven contributions to meteorology. For example, soar tional aircraft. Therefore, to gain or maintain ing pilots have probed thunderstorms and moun altitude, the soaring pilot must rely on upward tain waves with findings that have made flying motion of the air. safer for all pilots. However, soaring is primarily To a sailplane pilot, "lift" means the rate of recreational. climb he can achieve in an up-current, while "sink" A sailplane must have auxiliary power to be denotes his rate of descent in a downdraft or in come airborne such as a winch, a ground tow, or neutral air. "Zero sink" means that upward cur a tow by a powered aircraft. Once the sailcraft is rents are just strong enough to enable him to hold airborne and the tow cable released, performance altitude but not to climb. Sailplanes are highly 171 r efficient machines; a sink rate of a mere 2 feet per second. There is no point in trying to soar until second provides an airspeed of about 40 knots, and weather conditions favor vertical speeds greater a sink rate of 6 feet per second gives an airspeed than the minimum sink rate of the aircraft. -

Solar Thermal Energy
22 Solar Thermal Energy Solar thermal energy is an application of solar energy that is very different from photovol- taics. In contrast to photovoltaics, where we used electrodynamics and solid state physics for explaining the underlying principles, solar thermal energy is mainly based on the laws of thermodynamics. In this chapter we give a brief introduction to that field. After intro- ducing some basics in Section 22.1, we will discuss Solar Thermal Heating in Section 22.2 and Concentrated Solar (electric) Power (CSP) in Section 22.3. 22.1 Solar thermal basics We start this section with the definition of heat, which sometimes also is called thermal energy . The molecules of a body with a temperature different from 0 K exhibit a disordered movement. The kinetic energy of this movement is called heat. The average of this kinetic energy is related linearly to the temperature of the body. 1 Usually, we denote heat with the symbol Q. As it is a form of energy, its unit is Joule (J). If two bodies with different temperatures are brought together, heat will flow from the hotter to the cooler body and as a result the cooler body will be heated. Dependent on its physical properties and temperature, this heat can be absorbed in the cooler body in two forms, sensible heat and latent heat. Sensible heat is that form of heat that results in changes in temperature. It is given as − Q = mC p(T2 T1), (22.1) where Q is the amount of heat that is absorbed by the body, m is its mass, Cp is its heat − capacity and (T2 T1) is the temperature difference. -

Investigating Applicability of Evaporative Cooling Systems for Thermal Comfort of Poultry Birds in Pakistan
applied sciences Article Investigating Applicability of Evaporative Cooling Systems for Thermal Comfort of Poultry Birds in Pakistan Hafiz M. U. Raza 1, Hadeed Ashraf 1, Khawar Shahzad 1, Muhammad Sultan 1,* , Takahiko Miyazaki 2,3, Muhammad Usman 4,* , Redmond R. Shamshiri 5 , Yuguang Zhou 6 and Riaz Ahmad 6 1 Department of Agricultural Engineering, Bahauddin Zakariya University, Bosan Road, Multan 60800, Pakistan; [email protected] (H.M.U.R.); [email protected] (H.A.); [email protected] (K.S.) 2 Faculty of Engineering Sciences, Kyushu University, Kasuga-koen 6-1, Kasuga-shi, Fukuoka 816-8580, Japan; [email protected] 3 International Institute for Carbon-Neutral Energy Research (WPI-I2CNER), Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan 4 Institute for Water Resources and Water Supply, Hamburg University of Technology, Am Schwarzenberg-Campus 3, 20173 Hamburg, Germany 5 Leibniz Institute for Agricultural Engineering and Bioeconomy, Max-Eyth-Allee 100, 14469 Potsdam-Bornim, Germany; [email protected] 6 Bioenergy and Environment Science & Technology Laboratory, College of Engineering, China Agricultural University, Beijing 100083, China; [email protected] (Y.Z.); [email protected] (R.A.) * Correspondence: [email protected] (M.S.); [email protected] (M.U.); Tel.: +92-333-610-8888 (M.S.); Fax: +92-61-9210298 (M.S.) Received: 4 June 2020; Accepted: 24 June 2020; Published: 28 June 2020 Abstract: In the 21st century, the poultry sector is a vital concern for the developing economies including Pakistan. The summer conditions of the city of Multan (Pakistan) are not comfortable for poultry birds. -

Thermodynamics Formulation of Economics Burin Gumjudpai A
Thermodynamics Formulation of Economics 306706052 NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 Burin Gumjudpai A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Economics (Financial Economics) School of Development Economics National Institute of Development Administration 2019 Thermodynamics Formulation of Economics Burin Gumjudpai School of Development Economics Major Advisor (Associate Professor Yuthana Sethapramote, Ph.D.) The Examining Committee Approved This Thesis Submitted in Partial 306706052 Fulfillment of the Requirements for the Degree of Master of Economics (Financial Economics). Committee Chairperson NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 (Assistant Professor Pongsak Luangaram, Ph.D.) Committee (Assistant Professor Athakrit Thepmongkol, Ph.D.) Committee (Associate Professor Yuthana Sethapramote, Ph.D.) Dean (Associate Professor Amornrat Apinunmahakul, Ph.D.) ______/______/______ ABST RACT ABSTRACT Title of Thesis Thermodynamics Formulation of Economics Author Burin Gumjudpai Degree Master of Economics (Financial Economics) Year 2019 306706052 We consider a group of information-symmetric consumers with one type of commodity in an efficient market. The commodity is fixed asset and is non-disposable. We are interested in seeing if there is some connection of thermodynamics formulation NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 to microeconomics. We follow Carathéodory approach which requires empirical existence of equation of state (EoS) and coordinates before performing maximization of variables. In investigating EoS of various system for constructing the economics EoS, unexpectedly new insights of thermodynamics are discovered. With definition of truly endogenous function, criteria rules and diagrams are proposed in identifying the status of EoS for an empirical equation. -
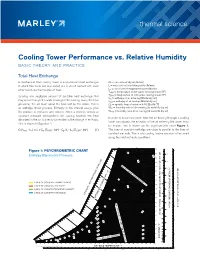
Thermal Science Cooling Tower Performance Vs. Relative Humidity
thermal science Cooling Tower Performance vs. Relative Humidity BASIC THEORY AND PRACTICE Total Heat Exchange A mechanical draft cooling tower is a specialized heat exchanger G = mass rate of dry air [lb/min] in which two fluids (air and water) are in direct contact with each L = mass rate of circulating water [lb/min] other to induce the transfer of heat. Le = mass rate of evaporated water [lb/min] THW = temperature of hot water entering tower [°F] Ignoring any negligible amount of sensible heat exchange that TCW = temperature of cold water leaving tower [°F] hin = enthalpy of air entering [Btu/lb/dry air] may occur through the walls (casing) of the cooling tower, the heat hout = enthalpy of air leaving [Btu/lb/dry air] gained by the air must equal the heat lost by the water. This is Cp = specific heat of water = 4.18 [Btu/lb-°F] an enthalpy driven process. Enthalpy is the internal energy plus Win = humidity ratio of air entering [lb water/lb dry air] the product of pressure and volume. When a process occurs at Wout = humidity ratio of air leaving [lb water/lb dry air] constant pressure (atmospheric for cooling towers), the heat In order to know how much heat the air flowing through a cooling absorbed in the air is directly correlated to the change in enthalpy. tower can absorb, the enthalpy of the air entering the tower must This is shown in Equation 1. be known. This is shown on the psychrometric chart Figure 1. G (hout - hin) = L x Cp (THW - 32°) - Cp (L - Le)(TCW - 32°) (1) The lines of constant enthalpy are close to parallel to the lines of constant wet bulb. -

Chapter 8 Atmospheric Statics and Stability
Chapter 8 Atmospheric Statics and Stability 1. The Hydrostatic Equation • HydroSTATIC – dw/dt = 0! • Represents the balance between the upward directed pressure gradient force and downward directed gravity. ρ = const within this slab dp A=1 dz Force balance p-dp ρ p g d z upward pressure gradient force = downward force by gravity • p=F/A. A=1 m2, so upward force on bottom of slab is p, downward force on top is p-dp, so net upward force is dp. • Weight due to gravity is F=mg=ρgdz • Force balance: dp/dz = -ρg 2. Geopotential • Like potential energy. It is the work done on a parcel of air (per unit mass, to raise that parcel from the ground to a height z. • dφ ≡ gdz, so • Geopotential height – used as vertical coordinate often in synoptic meteorology. ≡ φ( 2 • Z z)/go (where go is 9.81 m/s ). • Note: Since gravity decreases with height (only slightly in troposphere), geopotential height Z will be a little less than actual height z. 3. The Hypsometric Equation and Thickness • Combining the equation for geopotential height with the ρ hydrostatic equation and the equation of state p = Rd Tv, • Integrating and assuming a mean virtual temp (so it can be a constant and pulled outside the integral), we get the hypsometric equation: • For a given mean virtual temperature, this equation allows for calculation of the thickness of the layer between 2 given pressure levels. • For two given pressure levels, the thickness is lower when the virtual temperature is lower, (ie., denser air). • Since thickness is readily calculated from radiosonde measurements, it provides an excellent forecasting tool. -

Comparison Between Observed Convective Cloud-Base Heights and Lifting Condensation Level for Two Different Lifted Parcels
AUGUST 2002 NOTES AND CORRESPONDENCE 885 Comparison between Observed Convective Cloud-Base Heights and Lifting Condensation Level for Two Different Lifted Parcels JEFFREY P. C RAVEN AND RYAN E. JEWELL NOAA/NWS/Storm Prediction Center, Norman, Oklahoma HAROLD E. BROOKS NOAA/National Severe Storms Laboratory, Norman, Oklahoma 6 January 2002 and 16 April 2002 ABSTRACT Approximately 400 Automated Surface Observing System (ASOS) observations of convective cloud-base heights at 2300 UTC were collected from April through August of 2001. These observations were compared with lifting condensation level (LCL) heights above ground level determined by 0000 UTC rawinsonde soundings from collocated upper-air sites. The LCL heights were calculated using both surface-based parcels (SBLCL) and mean-layer parcels (MLLCLÐusing mean temperature and dewpoint in lowest 100 hPa). The results show that the mean error for the MLLCL heights was substantially less than for SBLCL heights, with SBLCL heights consistently lower than observed cloud bases. These ®ndings suggest that the mean-layer parcel is likely more representative of the actual parcel associated with convective cloud development, which has implications for calculations of thermodynamic parameters such as convective available potential energy (CAPE) and convective inhibition. In addition, the median value of surface-based CAPE (SBCAPE) was more than 2 times that of the mean-layer CAPE (MLCAPE). Thus, caution is advised when considering surface-based thermodynamic indices, despite the assumed presence of a well-mixed afternoon boundary layer. 1. Introduction dry-adiabatic temperature pro®le (constant potential The lifting condensation level (LCL) has long been temperature in the mixed layer) and a moisture pro®le used to estimate boundary layer cloud heights (e.g., described by a constant mixing ratio. -

Contribution of Horizontal Advection to the Interannual Variability of Sea Surface Temperature in the North Atlantic
964 JOURNAL OF PHYSICAL OCEANOGRAPHY VOLUME 33 Contribution of Horizontal Advection to the Interannual Variability of Sea Surface Temperature in the North Atlantic NATHALIE VERBRUGGE Laboratoire d'Etudes en GeÂophysique et OceÂanographie Spatiales, Toulouse, France GILLES REVERDIN Laboratoire d'Etudes en GeÂophysique et OceÂanographie Spatiales, Toulouse, and Laboratoire d'OceÂanographie Dynamique et de Climatologie, Paris, France (Manuscript received 9 February 2002, in ®nal form 15 October 2002) ABSTRACT The interannual variability of sea surface temperature (SST) in the North Atlantic is investigated from October 1992 to October 1999 with special emphasis on analyzing the contribution of horizontal advection to this variability. Horizontal advection is estimated using anomalous geostrophic currents derived from the TOPEX/ Poseidon sea level data, average currents estimated from drifter data, scatterometer-derived Ekman drifts, and monthly SST data. These estimates have large uncertainties, in particular related to the sea level product, the average currents, and the mixed-layer depth, that contribute signi®cantly to the nonclosure of the surface tem- perature budget. The large scales in winter temperature change over a year present similarities with the heat ¯uxes integrated over the same periods. However, the amplitudes do not match well. Furthermore, in the western subtropical gyre (south of the Gulf Stream) and in the subpolar regions, the time evolutions of both ®elds are different. In both regions, advection is found to contribute signi®cantly to the interannual winter temperature variability. In the subpolar gyre, advection often contributes more to the SST variability than the heat ¯uxes. It seems in particular responsible for a low-frequency trend from 1994 to 1998 (increase in the subpolar gyre and decrease in the western subtropical gyre), which is not found in the heat ¯uxes and in the North Atlantic Oscillation index after 1996. -

Potential Vorticity
POTENTIAL VORTICITY Roger K. Smith March 3, 2003 Contents 1 Potential Vorticity Thinking - How might it help the fore- caster? 2 1.1Introduction............................ 2 1.2WhatisPV-thinking?...................... 4 1.3Examplesof‘PV-thinking’.................... 7 1.3.1 A thought-experiment for understanding tropical cy- clonemotion........................ 7 1.3.2 Kelvin-Helmholtz shear instability . ......... 9 1.3.3 Rossby wave propagation in a β-planechannel..... 12 1.4ThestructureofEPVintheatmosphere............ 13 1.4.1 Isentropicpotentialvorticitymaps........... 14 1.4.2 The vertical structure of upper-air PV anomalies . 18 2 A Potential Vorticity view of cyclogenesis 21 2.1PreliminaryIdeas......................... 21 2.2SurfacelayersofPV....................... 21 2.3Potentialvorticitygradientwaves................ 23 2.4 Baroclinic Instability . .................... 28 2.5 Applications to understanding cyclogenesis . ......... 30 3 Invertibility, iso-PV charts, diabatic and frictional effects. 33 3.1 Invertibility of EPV ........................ 33 3.2Iso-PVcharts........................... 33 3.3Diabaticandfrictionaleffects.................. 34 3.4Theeffectsofdiabaticheatingoncyclogenesis......... 36 3.5Thedemiseofcutofflowsandblockinganticyclones...... 36 3.6AdvantageofPVanalysisofcutofflows............. 37 3.7ThePVstructureoftropicalcyclones.............. 37 1 Chapter 1 Potential Vorticity Thinking - How might it help the forecaster? 1.1 Introduction A review paper on the applications of Potential Vorticity (PV-) concepts by Brian