2004-201-DLD.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wilderness Advisory Committee Annual Report 2011-12
Wilderness Advisory Committee and Wilderness Protection Act 1992 ANNUAL REPORTS 1 July 2011 to 30 June 2012 Wilderness Advisory Committee Annual Report 2011-12 Wilderness Protection Act 1992 Annual Report 2011-12 For further information please contact: Executive Officer Wilderness Advisory Committee C/- Department of Environment, Water and Natural Resources GPO Box 1047 ADELAIDE SA 5001 Telephone: (08) 8124 4786 E-mail: [email protected] Website: www.environment.sa.gov.au ABN: 36 702 093 234 ISSN: 1832-9357 September 2012 _______________________________________________________________________________ Wilderness Advisory Committee Annual Report 2011-12 Page 2 TABLE OF CONTENTS LETTER OF TRANSMITTAL ....................................................................4 WILDERNESS ADVISORY COMMITTEE ANNUAL REPORT .................................5 PLANS AND OBJECTIVES ......................................................................5 OPERATIONS AND INITIATIVES ...............................................................5 ACHIEVEMENTS AND ACTIVITIES .........................................................5 WILDERNESS PROTECTION AREA MANAGEMENT PLANS .............................6 REQUESTS FOR ASSESSMENT TO THE WILDERNESS ADVISORY COMMITTEE .....7 ROLE, LEGISLATION AND STRUCTURE......................................................7 OBJECT OF THE WILDERNESS PROTECTION ACT .....................................7 ROLE OF THE COMMITTEE.................................................................7 FUNCTIONS OF THE WILDERNESS ADVISORY -

Cabinet Cover Sheet Title Protection of the White
CABINET COVER SHEET TITLE PROTECTION OF THE WHITE SHARK- REGULATIONS UNDER THE FISHERIES ACT 1982 AND THE NATIONAL PARKS AND WILDLIFE ACT 1972 MINISTERS HON DAVID WOTTON MP MINISTER FOR THE ENVIRONMENT AND NATURAL RESOURCES. HON ROB KERIN MP MINISTER FOR PRIMARY INDUSTRIES. PURPOSE To provide for the total protection of the white shark and to effect the management of the cage viewing charter industry. RESOURCES REQUIRED No cost implications to government FOR IMPLEMENTATION No effect on government staffing. RELATIONSHIP TO The proposal is consistent with government GOVERNMENT POLICY policy that the State's fisheries resources and habitat are managed on a sustainable basis. CONSULTATION Public consultation took place with the issue of a discussion paper and releases in the media. FAMILY IMPACT STATEMENT Not applicable. 8. URGENCY Not applicable. 9. RECOMMENDATIONS It is recommended that Cabinet approve - 4.1 The drafting of regulations to amend the Fisheries (General) Regulations 1984 as follows - 4.1.1 to proclaim the white shark {Carcharodon carcharias) a protected species under regulation 6, prohibiting the capture, holding or killing of this species; 4.1.2 to amend regulation 35C to: (a) prohibit the use of blood, bone, meat, offal or skin of an animal (other than in a rock lobster pot or fish trap) within two nautical miles of the mainland of the State or all islands and reefs of the State which are exposed at low water mark; and (b) prohibit the depositing of or use of a mammal or any product of a mammal in all marine waters of South Australia; 4.1.3 to prohibit the use of wire trace with a gauge of 2mm or greater, in conjunction with fishing hooks greater than size 12^/0, in all waters of South Australia. -

National Parks and Wildlife Act 1972.PDF
Version: 1.7.2015 South Australia National Parks and Wildlife Act 1972 An Act to provide for the establishment and management of reserves for public benefit and enjoyment; to provide for the conservation of wildlife in a natural environment; and for other purposes. Contents Part 1—Preliminary 1 Short title 5 Interpretation Part 2—Administration Division 1—General administrative powers 6 Constitution of Minister as a corporation sole 9 Power of acquisition 10 Research and investigations 11 Wildlife Conservation Fund 12 Delegation 13 Information to be included in annual report 14 Minister not to administer this Act Division 2—The Parks and Wilderness Council 15 Establishment and membership of Council 16 Terms and conditions of membership 17 Remuneration 18 Vacancies or defects in appointment of members 19 Direction and control of Minister 19A Proceedings of Council 19B Conflict of interest under Public Sector (Honesty and Accountability) Act 19C Functions of Council 19D Annual report Division 3—Appointment and powers of wardens 20 Appointment of wardens 21 Assistance to warden 22 Powers of wardens 23 Forfeiture 24 Hindering of wardens etc 24A Offences by wardens etc 25 Power of arrest 26 False representation [3.7.2015] This version is not published under the Legislation Revision and Publication Act 2002 1 National Parks and Wildlife Act 1972—1.7.2015 Contents Part 3—Reserves and sanctuaries Division 1—National parks 27 Constitution of national parks by statute 28 Constitution of national parks by proclamation 28A Certain co-managed national -

5 Day South Australian Wildlife Adventure Departs Thursdays (Weekly) and Saturdays (December to March)
KANGAROO ISLAND & EYRE PENINSULA 5 Day South Australian Wildlife Adventure Departs Thursdays (weekly) and Saturdays (December to March) 5 DAYS $1245pp SAVE $81 Enjoy all the must see and do experiences in two of South Australia’s most incredible wildlife and adventure regions, Kangaroo Island and the Eyre Peninsula on this great value, fi ve-day package. Tour with award winning tour companies, Kangaroo Island Adventure Tours and Australian Wildlife Adventures who will show you best parts of Australia you’ll ever see! 5 Day Package Includes: • Courtesy hostel pick-ups in Adelaide • Return coach and ferry transfers to Kangaroo Island • 2 days/1 night touring on Kangaroo Island with most meals • 3 days/2 nights touring around the Eyre Peninsula with most meals • 1 night hostel accommodation in Adelaide in-between tours 5 Days / 4 Nights ASK US $1245pp (normal price $1326) ABOUT Eyre SAVE $81 OUR PeninsulaP SPECIALS Departs Thursdays (weekly) and Saturdays KangarooKang (December to March) Island 2 DAYS KANGAROO ISLAND 3 DAYSDAYS EYREEYRE PENINSULA • Rob’s Sheep Shearing • Travel through Port Augusta to Port Lincoln – • Emu Ridge Eucalyptus Distillery seafood capital of Australia • Seal Bay Conservation Park (guided tour along • Hand feed and swim with Tuna (an amazing the beach amongst a colony of wild Australian marine wildlife encounter) Sealions) • Visit the koalas at Mikkira Station • Little Sahara sand dunes (for some wild • Whaler’s Way Conservation Park sand boarding) • Port Lincoln National Park bush walk • See koalas at Hanson Bay -

Great Australian Bight BP Oil Drilling Project
Submission to Senate Inquiry: Great Australian Bight BP Oil Drilling Project: Potential Impacts on Matters of National Environmental Significance within Modelled Oil Spill Impact Areas (Summer and Winter 2A Model Scenarios) Prepared by Dr David Ellis (BSc Hons PhD; Ecologist, Environmental Consultant and Founder at Stepping Stones Ecological Services) March 27, 2016 Table of Contents Table of Contents ..................................................................................................... 2 Executive Summary ................................................................................................ 4 Summer Oil Spill Scenario Key Findings ................................................................. 5 Winter Oil Spill Scenario Key Findings ................................................................... 7 Threatened Species Conservation Status Summary ........................................... 8 International Migratory Bird Agreements ............................................................. 8 Introduction ............................................................................................................ 11 Methods .................................................................................................................... 12 Protected Matters Search Tool Database Search and Criteria for Oil-Spill Model Selection ............................................................................................................. 12 Criteria for Inclusion/Exclusion of Threatened, Migratory and Marine -

Patterns of Future Developments in the South-West Marine Region
PATTERNS OF FUTURE DEVELOPMENT IN THE SOUTH-WEST MARINE REGION Julian Clifton, Maria Olejnik, Bryan Boruff and Matthew Tonts Institute for Regional Development December 2007 School of Earth & Geographical Sciences Prepared for the Australian Government Department of the Environment, Water, Heritage and the Arts ii © Commonwealth of Australia 2007 This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from the Commonwealth. Requests and inquiries concerning reproduction rights should be addressed to: Commonwealth Copyright Administration Attorney-General's Department Robert Garran Offices National Circuit Barton ACT 2600 or posted at http://www.ag.gov.au/cca. The Commonwealth Department of the Environment, Water, Heritage and the Arts commissioned the Institute for Regional Development, The University of Western Australia, School of Earth & Geographical Sciences to produce this publication. The views and opinions expressed in this publication are those of the authors and do not necessarily reflect those of the Australian Government or the Minister for the Environment, Water, Heritage and the Arts. The Commonwealth does not accept responsibility for the accuracy, completeness or currency of the contents, and shall not be liable for any loss or damage that may be occasioned directly or indirectly through the use of, or reliance on, the contents of this publication. i TABLE OF CONTENTS FIGURES AND TABLES vi LIST OF ABBREVIATIONS xi EXECUTIVE -

40 Great Short Walks
SHORT WALKS 40 GREAT Notes SOUTH AUSTRALIAN SHORT WALKS www.southaustraliantrails.com 51 www.southaustraliantrails.com www.southaustraliantrails.com NORTHERN TERRITORY QUEENSLAND Simpson Desert Goyders Lagoon Macumba Strzelecki Desert Creek Sturt River Stony Desert arburton W Tirari Desert Creek Lake Eyre Cooper Strzelecki Desert Lake Blanche WESTERN AUSTRALIA WESTERN Outback Great Victoria Desert Lake Lake Flinders Frome ALES Torrens Ranges Nullarbor Plain NORTHERN TERRITORY QUEENSLAND Simpson Desert Goyders Lagoon Lake Macumba Strzelecki Desert Creek Gairdner Sturt 40 GREAT SOUTH AUSTRALIAN River Stony SHORT WALKS Head Desert NEW SOUTH W arburton of Bight W Trails Diary date completed Trails Diary date completed Tirari Desert Creek Lake Gawler Eyre Cooper Strzelecki ADELAIDE Desert FLINDERS RANGES AND OUTBACK 22 Wirrabara Forest Old Nursery Walk 1 First Falls Valley Walk Ranges QUEENSLAND A 2 First Falls Plateau Hike Lake 23 Alligator Gorge Hike Blanche 3 Botanic Garden Ramble 24 Yuluna Hike Great Victoria Desert 4 Hallett Cove Glacier Hike 25 Mount Ohlssen Bagge Hike Great Eyre Outback 5 Torrens Linear Park Walk 26 Mount Remarkable Hike 27 The Dutchmans Stern Hike WESTERN AUSTRALI WESTERN Australian Peninsula ADELAIDE HILLS 28 Blinman Pools 6 Waterfall Gully to Mt Lofty Hike Lake Bight Lake Frome ALES 7 Waterfall Hike Torrens KANGAROO ISLAND 0 50 100 Nullarbor Plain 29 8 Mount Lofty Botanic Garden 29 Snake Lagoon Hike Lake 25 30 Weirs Cove Gairdner 26 Head km BAROSSA NEW SOUTH W of Bight 9 Devils Nose Hike LIMESTONE COAST 28 Flinders -

(Haliaeetus Leucogaster) and the Eastern Osprey (Pandion Cristatus
SOUTH AUSTRALIAN ORNITHOLOGIST VOLUME 37 - PART 1 - March - 2011 Journal of The South Australian Ornithological Association Inc. In this issue: Osprey and White-bellied Sea-Eagle populations in South Australia Birds of Para Wirra Recreation Park Bird report 2009 March 2011 1 Distribution and status of White-bellied Sea-Eagle, Haliaeetus leucogaster, and Eastern Osprey, Pandion cristatus, populations in South Australia T. E. DENNIS, S. A. DETmAR, A. V. BROOkS AND H. m. DENNIS. Abstract Surveys throughout coastal regions and in the INTRODUCTION Riverland of South Australia over three breeding seasons between May 2008 and October 2010, Top-order predators, such as the White-bellied estimated the population of White-bellied Sea- Sea-Eagle, Haliaeetus leucogaster, and Eastern Eagle, Haliaeetus leucogaster, as 70 to 80 pairs Osprey, Pandion cristatus, are recognised and Eastern Osprey, Pandion cristatus, as 55 to indicator species by which to measure 65 pairs. Compared to former surveys these data wilderness quality and environmental integrity suggest a 21.7% decline in the White-bellied Sea- in a rapidly changing world (Newton 1979). In Eagle population and an 18.3% decline for Eastern South Australia (SA) both species have small Osprey over former mainland habitats. Most (79.2%) populations with evidence of recent declines sea-eagle territories were based on offshore islands linked to increasing human activity in coastal including Kangaroo Island, while most (60.3%) areas (Dennis 2004; Dennis et al. 2011 in press). osprey territories were on the mainland and near- A survey of the sea-eagle population in the shore islets or reefs. The majority of territories were mid 1990s found evidence for a decline in the in the west of the State and on Kangaroo Island, with breeding range since European colonisation three sub-regions identified as retaining significant (Dennis and Lashmar 1996). -

Research Report Series
GREAT AUSTRALIAN BIGHT RESEARCH PROGRAM RESEARCH REPORT SERIES Status, distribution and abundance of iconic species and apex predators in the Great Australian Bight Final Report GABRP Project 4.1 S.D. Goldsworthy, A.I. MacKay, K. Bilgmannn, L.M. Möller, G.J. Parra, P. Gill, F. Bailleul, P. Shaughnessy, S-L. Reinhold and P.J. Rogers. GABRP Research Report Number 15 October 2017 DISCLAIMER The partners of the Great Australian Bight Research Program advises that the information contained in this publication comprises general statements based on scientific research. The reader is advised that no reliance or actions should be made on the information provided in this report without seeking prior expert professional, scientific and technical advice. To the extent permitted by law, the partners of the Great Australian Bight Research Program (including its employees and consultants) excludes all liability to any person for any consequences, including but not limited to all losses, damages, costs, expenses and any other compensation, arising directly or indirectly from using this publication (in part or in whole) and any information or material contained in it. The GABRP Research Report Series is an Administrative Report Series which has not been reviewed outside the Great Australian Bight Research Program and is not considered peer-reviewed literature. Material presented may later be published in formal peer-reviewed scientific literature. COPYRIGHT ©2017 THIS PUBLICATION MAY BE CITED AS: Goldsworthy S.D, Mackay A.I, Bilgmann K, MÖller L.M, Parra G.J, Gill P, Bailleul F, Shaughnessy P, Reinhold S-L and Rogers P.J (2017). Status, distribution and abundance of iconic species and apex predators in the Great Australian Bight. -
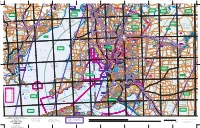
S P E N C E R G U L F S T G U L F V I N C E N T Adelaide
Yatala Harbour Paratoo Hill Turkey 1640 Sunset Hill Pekina Hill Mt Grainger Nackara Hill 1296 Katunga Booleroo "Avonlea" 2297 Depot Hill Creek 2133 Wilcherry Hill 975 Roopena 1844 Grampus Hill Anabama East Hut 1001 Dawson 1182 660 Mt Remarkable SOUTH Mount 2169 440 660 (salt) Mt Robert Grainger Scobie Hill "Mazar" vermin 3160 2264 "Manunda" Wirrigenda Hill Weednanna Hill Mt Whyalla Melrose Black Rock Goldfield 827 "Buckleboo" 893 729 Mambray Creek 2133 "Wyoming" salt (2658±) RANGE Pekina Wheal Bassett Mine 1001 765 Station Hill Creek Manunda 1073 proof 1477 Cooyerdoo Hill Maurice Hill 2566 Morowie Hill Nackara (abandoned) "Bulyninnie" "Oak Park" "Kimberley" "Wilcherry" LAKE "Budgeree" fence GILLES Booleroo Oratan Rock 417 Yeltanna Hill Centre Oodla "Hill Grange" Plain 1431 "Gilles Downs" Wirra Hillgrange 1073 B pipeline "Wattle Grove" O Tcharkuldu Hill T Fullerville "Tiverton 942 E HWY Outstation" N Backy Pt "Old Manunda" 276 E pumping station L substation Tregalana Baroota Yatina L Fitzgerald Bay A Middleback Murray Town 2097 water Ucolta "Pitcairn" E Buckleboo 1306 G 315 water AN Wild Dog Hill salt Tarcowie R Iron Peak "Terrananya" Cunyarie Moseley Nobs "Middleback" 1900 works (1900±) 1234 "Lilydale" H False Bay substation Yaninee I Stoney Hill O L PETERBOROUGH "Blue Hills" LC L HWY Point Lowly PEKINA A 378 S Iron Prince Mine Black Pt Lancelot RANGE (2294±) 1228 PU 499 Corrobinnie Hill 965 Iron Baron "Oakvale" Wudinna Hill 689 Cortlinye "Kimboo" Iron Baron Waite Hill "Loch Lilly" 857 "Pualco" pipeline Mt Nadjuri 499 Pinbong 1244 Iron -

Monitoring of Cape Barren Goose Populations in South Australia
184 SOUTH AUSTRALIAN ORNITHOLOGIST, 30 MONITORING OF CAPE BARREN GOOSE POPULATIONS IN SOUTH AUSTRALIA. II. THE 1987 BREEDING SEASON AND FURTHER BANDING RECOVERIES L. B. DELROY, A. C. ROBINSON AND M. H. WATERMAN INTRODUCTION to limitations on boat availability. On 21 to 23 July 1987four observers landed on 10islands in The South Australian National Parks and the Sir Joseph Banks Group and systematic Wildlife Service conducted ecological studies of counts of adults, chicks and eggs of Cape Barren the Cape Barren Goose Cereopsis novaehollan Geese on each island were undertaken. The diae in its breeding habitat in 1974, 1979and 1985 observers made successive parallel sweeps, with and two surveys of its summering habitats in 1979 one member using a compass, until the whole and 1985. These surveys involved counts of island had been completely covered. Observers adults, chicks, eggs and nests in the major counted within approximately a 30 metre wide breeding habitat of the Cape Barren Goose in the strip, zig zagging constantly within the strip so Sir Joseph Banks Group islands offshore from that the distance of scan was as low as two or Port Lincoln, and the monitoring of the three metres in dense vegetation or where summering habitat around lakes Alexandrina and numbers were high, but up to 15 metres in open Albert. Details of this work have been reported country. This level of observation was in Robinson et at. (1982) and Robinson & Delroy approximately the same as that used in previous (1986). surveys. As the line moved across an island, all In the 1985 survey of the Sir Joseph Banks nests sighted were inspected for eggs (and to Group, rainfall in theIate autumn and early ensure that they contained down and were not winter had been low and numbers of adult birds, nests from previous years) and chicks were eggsand chicks wereless than in the two previous recorded as they were seen. -

Preserving the West Coast of South Australia 2 Contents
WRITTEN BY DAVID LETCH Chain of Bays PHOTOGRAPHY BY GRANT HOBSON Preserving the West Coast of South Australia 2 Contents chapter 1 Preserving a unique coastal area 5 chapter 2 The Wirangu people 11 chapter 3 Living in a wild coastal ecosystem 17 chapter 4 Scientists, surfers, naturalists & tourists 21 chapter 5 Regulating impacts on nature 25 chapter 6 Tyringa & Baird Bay 31 chapter 7 Searcy Bay 37 chapter 8 Sceale Bay 41 chapter 9 Corvisart Bay 47 chapter 10 Envisaging the long term 49 chapter 11 Local species lists 51 chapter 12 Feedback & getting involved in conservation 55 chapter 12 References 57 chapter 12 Acknowledgements 59 Front cover image: Alec Baldock and Juvenile Basking Shark (1990). The taxonomy and traits of many species can remain a mystery. This image was sent to the Melbourne Museum where the species was identified - a rare image collected locally. Back cover image: Crop surrounding a pocket of native vegetation (2009). Much land has been cleared for farming in the Chain of Bays. Small tracts of native vegetation represent opportunities for seed collection and habitat preservation. Connecting these micro habitats is the real challenge. Inside cover: Cliff top vegetation Tyringa (2009). In the Chain of Bays sensitive vegetation clings to the calciferous limestone cliffs. Off road vehicles and quad bikes pose an increasing threat in the Chain of Bays. Right image: Death Adder Sceale Bay (2010). These beautiful and highly venomous reptiles are very rarely seen by local people suggesting their numbers may be low in the area.