Chapter 9 Non-Aqueous Media
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wastewater Technology Fact Sheet: Ammonia Stripping
United States Office of Water EPA 832-F-00-019 Environmental Protection Washington, D.C. September 2000 Agency Wastewater Technology Fact Sheet Ammonia Stripping DESCRIPTION Ammonia stripping is a simple desorption process used to lower the ammonia content of a wastewater stream. Some wastewaters contain large amounts of ammonia and/or nitrogen-containing compounds that may readily form ammonia. It is often easier and less expensive to remove nitrogen from wastewater in the form of ammonia than to convert it to nitrate-nitrogen before removing it (Culp et al., 1978). Ammonia (a weak base) reacts with water (a weak acid) to form ammonium hydroxide. In ammonia stripping, lime or caustic is added to the wastewater until the pH reaches 10.8 to 11.5 standard units which converts ammonium hydroxide ions to ammonia gas according to the following reaction(s): + - NH4 + OH 6 H2O + NH38 Source: Culp, et. al, 1978. Figure 1 illustrates two variations of ammonia FIGURE 1 TWO TYPES OF STRIPPING stripping towers, cross-flow and countercurrent. In TOWERS a cross-flow tower, the solvent gas (air) enters along the entire depth of fill and flows through the packing, as the alkaline wastewater flows it may be more economical to use alternate downward. A countercurrent tower draws air ammonia removal techniques, such as steam through openings at the bottom, as wastewater is stripping or biological methods. Air stripping may pumped to the top of a packed tower. Free also be used to remove many hydrophobic organic ammonia (NH3) is stripped from falling water molecules (Nutrient Control, 1983). droplets into the air stream, then discharged to the atmosphere. -

Understanding Variation in Partition Coefficient, Kd, Values: Volume II
United States Office of Air and Radiation EPA 402-R-99-004B Environmental Protection August 1999 Agency UNDERSTANDING VARIATION IN PARTITION COEFFICIENT, Kd, VALUES Volume II: Review of Geochemistry and Available Kd Values for Cadmium, Cesium, Chromium, Lead, Plutonium, Radon, Strontium, Thorium, Tritium (3H), and Uranium UNDERSTANDING VARIATION IN PARTITION COEFFICIENT, Kd, VALUES Volume II: Review of Geochemistry and Available Kd Values for Cadmium, Cesium, Chromium, Lead, Plutonium, Radon, Strontium, Thorium, Tritium (3H), and Uranium August 1999 A Cooperative Effort By: Office of Radiation and Indoor Air Office of Solid Waste and Emergency Response U.S. Environmental Protection Agency Washington, DC 20460 Office of Environmental Restoration U.S. Department of Energy Washington, DC 20585 NOTICE The following two-volume report is intended solely as guidance to EPA and other environmental professionals. This document does not constitute rulemaking by the Agency, and cannot be relied on to create a substantive or procedural right enforceable by any party in litigation with the United States. EPA may take action that is at variance with the information, policies, and procedures in this document and may change them at any time without public notice. Reference herein to any specific commercial products, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government. ii FOREWORD Understanding the long-term behavior of contaminants in the subsurface is becoming increasingly more important as the nation addresses groundwater contamination. Groundwater contamination is a national concern as about 50 percent of the United States population receives its drinking water from groundwater. -

Electric Permittivity of Carbon Fiber
Carbon 143 (2019) 475e480 Contents lists available at ScienceDirect Carbon journal homepage: www.elsevier.com/locate/carbon Electric permittivity of carbon fiber * Asma A. Eddib, D.D.L. Chung Composite Materials Research Laboratory, Department of Mechanical and Aerospace Engineering, University at Buffalo, The State University of New York, Buffalo, NY, 14260-4400, USA article info abstract Article history: The electric permittivity is a fundamental material property that affects electrical, electromagnetic and Received 19 July 2018 electrochemical applications. This work provides the first determination of the permittivity of contin- Received in revised form uous carbon fibers. The measurement is conducted along the fiber axis by capacitance measurement at 25 October 2018 2 kHz using an LCR meter, with a dielectric film between specimen and electrode (necessary because an Accepted 11 November 2018 LCR meter is not designed to measure the capacitance of an electrical conductor), and with decoupling of Available online 19 November 2018 the contributions of the specimen volume and specimen-electrode interface to the measured capaci- tance. The relative permittivity is 4960 ± 662 and 3960 ± 450 for Thornel P-100 (more graphitic) and Thornel P-25 fibers (less graphitic), respectively. These values are high compared to those of discon- tinuous carbons, such as reduced graphite oxide (relative permittivity 1130), but are low compared to those of steels, which are more conductive than carbon fibers. The high permittivity of carbon fibers compared to discontinuous carbons is attributed to the continuity of the fibers and the consequent substantial distance that the electrons can move during polarization. The P-100/P-25 permittivity ratio is 1.3, whereas the P-100/P-25 conductivity ratio is 67. -

Properties of Acids and Bases
GREEN CHEMISTRY LABORATORY MANUAL Lab 22 Properties of Acids and Bases TN Standard 4.2: The student will investigate the characteristics of acids and bases. Have you ever brushed your teeth and then drank a glass of orange juice? hat do you taste when you brush your teeth and drink orange juice afterwards. Yuck! It leaves a really bad taste in your mouth, but why? Orange juice and toothpaste by themselves taste good. But the terrible taste W results because an acid/base reaction is going on in your mouth. Orange juice is a weak acid and the toothpaste is a weak base. When they are placed together they neutralize each other and produce a product that is unpleasant to taste. How do you determine what is an acid and what is a base? In this lab we will discover how to distinguish between acids and bases. Introduction Two very important classes of compounds are acids and bases. But what exactly makes them different? There are differences in definition, physical differences, and reaction differences. According to the Arrhenius definition, acids ionize in water to + produce a hydronium ion (H3O ), and bases dissociate in water to produce hydroxide ion (OH -). Physical differences can be detected by the senses, including taste and touch. Acids have a sour or tart taste and can produce a stinging sensation to broken skin. For example, if you have ever tasted a lemon, it can often result in a sour face. Bases have a bitter taste and a slippery feel. Soap and many cleaning products are bases. -

VELOCITY of PROPAGATION by RON HRANAC
Originally appeared in the March 2010 issue of Communications Technology. VELOCITY OF PROPAGATION By RON HRANAC If you’ve looked at a spec sheet for coaxial cable, you’ve no doubt seen a parameter called velocity of propagation. For instance, the published velocity of propagation for CommScope’s F59 HEC-2 headend cable is "84% nominal," and Times Fiber’s T10.500 feeder cable has a published value of "87% nominal." What do these numbers mean, and where do they come from? We know that the speed of light in free space is 299,792,458 meters per second, which works out to 299,792,458/0.3048 = 983,571,056.43 feet per second, or 983,571,056.43/5,280 = 186,282.4 miles per second. The reciprocal of the free space value of the speed of light in feet per second is the time it takes for light to travel 1 foot: 1/983,571,056.43 = 1.02E-9 second, or 1.02 nanosecond. In other words, light travels a foot in free space in about a billionth of a second. Light is part of the electromagnetic spectrum, as is RF. That means RF zips along at the same speed that light does. "The major culprit that slows the waves down is the dielectric — and it slows TEM waves down a bunch." Now let’s define velocity of propagation: It’s the speed at which an electromagnetic wave propagates through a medium such as coaxial cable, expressed as a percentage of the free space value of the speed of light. -

Importance of Varying Permittivity on the Conductivity of Polyelectrolyte Solutions
Importance of Varying Permittivity on the Conductivity of Polyelectrolyte Solutions Florian Fahrenberger, Owen A. Hickey, Jens Smiatek, and Christian Holm∗ Institut f¨urComputerphysik, Universit¨atStuttgart, Allmandring 3, Stuttgart 70569, Germany (Dated: September 8, 2018) Dissolved ions can alter the local permittivity of water, nevertheless most theories and simulations ignore this fact. We present a novel algorithm for treating spatial and temporal variations in the permittivity and use it to measure the equivalent conductivity of a salt-free polyelectrolyte solution. Our new approach quantitatively reproduces experimental results unlike simulations with a constant permittivity that even qualitatively fail to describe the data. We can relate this success to a change in the ion distribution close to the polymer due to the built-up of a permittivity gradient. The dielectric permittivity " measures the polarizabil- grained approach is necessary due to the excessive sys- ity of a medium subjected to an electric field and is one of tem size. Molecular Dynamics (MD) simulations of only two fundamental constants in Maxwell's equations. charged systems typically work with the restricted prim- The relative permittivity of pure water at room temper- itive model by simulating the ions as hard spheres while ature is roughly 78.5, but charged objects dissolved in accounting for the solvent implicitly through a con- the fluid significantly reduce the local dielectric constant stant background dielectric. Crucially, the solvent me- because water dipoles align with the local electric field diates hydrodynamic interactions and reduces the elec- created by the object rather than the external field [1{3]. trostatic interactions due to its polarizability. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

Chapter 4: Aqueous Solutions (Chs 4 and 5 in Jespersen, Ch4 in Chang)
Chapter 4: Aqueous Solutions (Chs 4 and 5 in Jespersen, Ch4 in Chang) Solutions in which water is the dissolving medium are called aqueous solutions. There are three major types of chemical processes occurring in aqueous solutions: precipitation reactions (insoluble product) acid-base reactions (transfer of H+s) redox reactions (transfer of electrons) General Properties of Aqueous Solutions solution – a homogeneous mixture of two or more substances. solvent – usually the component that is present in greater quantity. solute(s) – the other substance(s) in the solution (ionic or molecular); present in smaller quantities. saturated solution – at a given temperature, the solution that results when the maximum amount of a substance has dissolved in a solvent. AJR Ch4 Aqueous Solutions.docx Slide 1 Electrolytic Properties electrolyte – a substance whose aqueous solutions contains ions and hence conducts electricity. E.g. NaCl. nonelectrolyte – a substance that does not form ions when it dissolves in water, and so aqueous solutions of nonelectrolytes do not conduct electricity. E.g. Glucose, C6H12O6. CuSO4 and Water; Ions present; Sugar and Water; No Ions present; electricity is conducted – bulb on. electricity is not conducted – bulb off. AJR Ch4 Aqueous Solutions.docx Slide 2 Ionic Compounds in Water Ionic compounds dissociate into their component ions as they dissolve in water. The ions become hydrated. Each ion is surrounded by water molecules, with the negative pole of the water oriented towards the cation and the positive pole oriented towards the anion. Other examples of ionic compounds dissociating into their component ions: + 2– Na2CO3 → 2 Na (aq) + CO3 (aq) + 2– (NH4)2SO4 → 2 NH4 (aq) + SO4 (aq) AJR Ch4 Aqueous Solutions.docx Slide 3 Molecular Compounds in Water Most molecular compounds do not form ions when they dissolve in water; they are nonelectrolytes. -
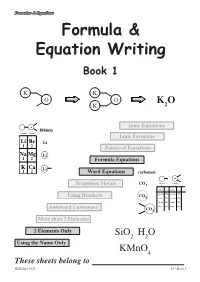
Formula & Equation Writing
Formulae & Equations Formula & Equation Writing Book 1 K K O O K O K 2 Ionic Equations lithium column column 1 2 Ionic Formulae Li Be Li 1 2 Balanced Equations Na Mg Li 1 2 Formula Equations K Ca Li 1 2 Word Equations carbonate valency valency Transition Metals CO3 1 2 name formula name formula ammonium NH4 carbonate CO3 Using Brackets CO 3 cyanide CN chromate CrO4 hydroxide OH sulphate SO4 nitrate NO sulphite SO Awkward Customers 3 3 CO3 More than 2 Elements 2 Elements Only SiO2 H2O Using the Name Only KMnO4 These sheets belong to KHS Sept 2013 page 1 S3 - Book 1 Formulae & Equations What is a Formula ? The formula of a compound tells you two things about the compound :- SiO H O i) which elements are in the compound 2 2 using symbols, and KMnO4 ii) how many atoms of each element are in the compound using numbers. Test Yourself What would be the formula for each of the following? Br H Cl K H C S Al Br O H Cl K H Br Using the Name Only Some compounds have extra information in their carbon monoxide names that allow people to work out and write CO the correct formula. carbon dioxide CO2 The names of the elements appear as usual but dinitrogen tetroxide N O this time the number of each type of atom is 2 4 included using mono- = 1 di- = 12 tri- = 3 tetra- = 4 penta- = 5 hexa- =46 Test Yourself 1 What would be the formula for each of the following? 1. -

Drugs and Acid Dissociation Constants Ionisation of Drug Molecules Most Drugs Ionise in Aqueous Solution.1 They Are Weak Acids Or Weak Bases
Drugs and acid dissociation constants Ionisation of drug molecules Most drugs ionise in aqueous solution.1 They are weak acids or weak bases. Those that are weak acids ionise in water to give acidic solutions while those that are weak bases ionise to give basic solutions. Drug molecules that are weak acids Drug molecules that are weak bases where, HA = acid (the drug molecule) where, B = base (the drug molecule) H2O = base H2O = acid A− = conjugate base (the drug anion) OH− = conjugate base (the drug anion) + + H3O = conjugate acid BH = conjugate acid Acid dissociation constant, Ka For a drug molecule that is a weak acid The equilibrium constant for this ionisation is given by the equation + − where [H3O ], [A ], [HA] and [H2O] are the concentrations at equilibrium. In a dilute solution the concentration of water is to all intents and purposes constant. So the equation is simplified to: where Ka is the acid dissociation constant for the weak acid + + Also, H3O is often written simply as H and the equation for Ka is usually written as: Values for Ka are extremely small and, therefore, pKa values are given (similar to the reason pH is used rather than [H+]. The relationship between pKa and pH is given by the Henderson–Hasselbalch equation: or This relationship is important when determining pKa values from pH measurements. Base dissociation constant, Kb For a drug molecule that is a weak base: 1 Ionisation of drug molecules. 1 Following the same logic as for deriving Ka, base dissociation constant, Kb, is given by: and Ionisation of water Water ionises very slightly. -

Measurement of Dielectric Material Properties Application Note
with examples examples with solutions testing practical to show written properties. dielectric to the s-parameters converting for methods shows Italso analyzer. a network using materials of properties dielectric the measure to methods the describes note The application | | | | Products: Note Application Properties Material of Dielectric Measurement R&S R&S R&S R&S ZNB ZNB ZVT ZVA ZNC ZNC Another application note will be will note application Another <Application Note> Kuek Chee Yaw 04.2012- RAC0607-0019_1_4E Table of Contents Table of Contents 1 Overview ................................................................................. 3 2 Measurement Methods .......................................................... 3 Transmission/Reflection Line method ....................................................... 5 Open ended coaxial probe method ............................................................ 7 Free space method ....................................................................................... 8 Resonant method ......................................................................................... 9 3 Measurement Procedure ..................................................... 11 4 Conversion Methods ............................................................ 11 Nicholson-Ross-Weir (NRW) .....................................................................12 NIST Iterative...............................................................................................13 New non-iterative .......................................................................................14 -

Nitric Acid Plants (PDF)
Office of Air and Radiation December 2010 AVAILABLE AND EMERGING TECHNOLOGIES FOR REDUCING GREENHOUSE GAS EMISSIONS FROM THE NITRIC ACID PRODUCTION INDUSTRY Available and Emerging Technologies for Reducing Greenhouse Gas Emissions from the Nitric Acid Production Industry Prepared by the Sector Policies and Programs Division Office of Air Quality Planning and Standards U.S. Environmental Protection Agency Research Triangle Park, North Carolina 27711 December 2010 Table of Contents Abbreviations and Acronyms p.1 I Introduction /Purpose of this Document p.2 II. Description of the Nitric Acid Production Process p.3 A. Weak Nitric Acid Production p.3 B. High-Strength Nitric Acid Production p.6 III. N2O Emissions and Nitric Acid Production Process p. 7 IV. Summary of Control Measures p.9 A. Primary Controls p.10 B. Secondary Control p.11 C. Tertiary Controls p.13 D. Selective Catalytic Reduction p.16 V. Other Greenhouse Gas Emissions p.16 VI. Energy Efficiency Improvements p.17 VII. EPA Contacts p.19 VIII. References p.20 Appendix A – Southeast Idaho Energy p.23 Appendix B - US Nitric Acid Plants p.24 Appendix C – CDM Monitoring Reports p.25 Abbreviations and Acronyms atm Pressure in atmospheres BACT Best Available Control Technologies Btu British Thermal Unit Btu/lb Btu per lb of 100% nitric acid CAR Climate Action Reserve CDM Clean Development Mechanism CHP Combined Heat and Power CO2 Carbon dioxide CO2e Carbon dioxide equivalent EU European Union GHG Greenhouse Gases H2 Hydrogen IPCC Intergovernmental Panel on Climate Change IPPC Industrial Pollution Prevention and Control JI Joint Implementation kg N2O/tonne kilograms of N2O per tonne of 100% nitric acid kg CO2e/tonne kilograms of CO2e per tonne of 100% nitric acid lb N2O/ton pounds of N2O per ton of 100% nitric acid N2 Nitrogen NH3 Ammonia NO Nitric oxide NO2 Nitrogen dioxide NOx Nitrogen oxides NSCR Nonselective Catalytic Reduction N2 O4 Nitrogen tetraoxide N2O Nitrous oxide O2 Oxygen PSD Prevention of Significant Deterioration SCR Selective Catalytic Reduction TPD Tons per day 1 I.